Article
Bioavailability of a Nanoemulsion of Lutein Is Greater than a Lutein Supplement
Rohini Vishwanathan, Thomas A. Wilson, and Robert J. Nicolosi *
Department of Clinical Laboratory and Nutritional Sciences, Center for Health and Disease Research, University of Massachusetts Lowell, Lowell, MA
* Corresponding author. Email: nicolosi.robert@yahoo.com
Citation: R. Vishwanathan, et al., Bioavailability of a Nanoemulsion of Lutein Is Greater than a Lutein Supplement. Nano Biomed. Eng., 2009, 1(1): 38-49.
DOI: 10.5101/nbe.v1i1.p38-49
Abstract
Lutein, a lipid soluble, oxygenated carotenoid, has shown beneficial effects against the risk factors associated with age-related macular degeneration, cardiovascular disease and also damaging UV radiation. The goal of the present study was to formulate lutein into a stable hydrophilic nanoemulsion that is more bioavailable and consumable in a matrix such as a beverage rather than just supplements. A Microfluidizer® Processor was used to convert an oil-in-water lutein emulsion into a nanoemulsion that is a stable water dispersion and measures 150 nm. After a one wk baseline phase, subjects consumed a lutein supplement pill followed by a lutein nanoemulsion added to orange juice (6 mg/d and 2 mg/d in two separate studies) for one wk each with a 2 wk washout phase between treatments. In study 1, mean serum lutein concentrations (n = 9) increased by 104% (P < 0.001) and 167% (P < 0.001) after the 6 mg supplement and nanoemulsion phases, respectively. In study 2, mean serum lutein concentrations (n = 11) increased by 37% (P < 0.05) and 75% (P < 0.001) after the 2 mg lutein supplement and nanoemulsion phases, respectively Despite the fact that the actual concentration of lutein in the 6 mg and 2 mg nanoemulsions was 10% and 40% lower compared to the supplement form, respectively, due to Microfluidizer® processor preparation loss, the nanoemulsions resulted in 31% (P < 0.05) and 28% (P < 0.05) greater serum lutein concentrations compared to the supplement.. In conclusion, nanoemulsions of lutein had significantly greater bioavailability than the supplement-pill forms.
Keywords: Lutein; Nanoemulsions; Supplements; Agerelated macular degeneration; Macular pigment; Bioavailability
1 Introduction
Lutein, an oxygenated carotenoid protects against age-related macular degeneration (AMD), the leading cause of vision loss in a population aged 60 y and older [1, 2]. Lutein together with zeaxanthin and meso- zeaxanthin forms macular pigment, which filters dam- aging blue (450 nm) radiation of sunlight from reaching the retinal cells [3]. Lutein being an antioxidant also protects the retinal pigment epithelial cells from reactive oxygen species [4]. Lutein may also reduce the risk of coronary heart disease and protect the skin from UV induced damage [5]. Digestibility of the food matrix carrying lutein and its dissolution in lipid micelles affects bioavailability of lutein, which makes plant sources less bioavailable un- less processed and/or consumed with a minimum amount of fat [6, 7]. Egg yolks as a source of lutein are more bioavailable than spinach or lutein supplements [8]. The increased bioavailability of lutein from egg yolks may be attributed to the natural micellar matrix of cholesterol (200 mg/yolk), triglycerides (4 g/yolk) and phospholipids (1 g/yolk) [9]. Egg yolks have been re- ported to contain an average of 250 to 500 µg of lutein and zeaxanthin per yolk [10-11]. The egg yolk micellar particles carrying lutein are 1-2 microns in size (data not shown). In general smaller particle sizes, in the nano range (50 - 200 nm) of personal care and cosmetic for- mulations have been associated with increased bioavailability due to a greater surface to volume ratio [12]. Nanoformulations are also kinetically stable, and when adequately prepared can have a long shelf life [12]. Our lutein nanoemulsion generated by Microfluidizer® Processor technology (patent pending) is a cholesterol free, oil-in-water emulsion, with particle sizes ranging from 100 - 150 nm, comprised of soybean oil and a phospho- lipid emulsifier that converts the normally hydrophobic lutein molecule into a stable water dispersion. Nanoemulsions have a hydrophobic core unlike lipo- somes, which have an aqueous core due to the bilayer configuration of polar lipids that are used to form liposomes [13]. Our laboratory has successfully formulated nutrients such as delta-tocopherol [14] and gamma- tocopherol [15] into stable nanoemulsion water dispersions and demonstrated increased bioavailability com- pared to microemulsions using animal models. In addi- tion, other labs using nano preparations of paclitaxel [16], as well as our reported studies have shown in- creased bioavailability and efficacy of nanoformulations of anti-cancer drugs such as tamoxifen [17] and dacarbazine [18] and anti-inflammatory compounds such as aspirin [19] compared to micro-suspensions using cell culture and animal models. The objective of this study was to develop (a) a stable water dispersion of lutein that can be added to a beverage and used in addition to or in place of the sup- plement pill form especially, in an AMD-susceptible older adult population who presently consume lutein supplement pills (b) a nanoemulsion delivery system for lutein that has greater bioavailability than supplement- pill form of lutein at equivalent doses. Two forms and doses of lutein: a 6 mg lutein supplement pill (Study 1) and a multivitamin pill containing 2 mg lutein (Study 2) were compared to 6 mg and 2 mg lutein nanoemulsions, respectively, in two separate studies in healthy adults aged 18 y and older.
2 Materials and Methods
2.1 Subjects
Recruitment was done at the Center for Health and Disease Research, University of Massachusetts Lowell. Staff and students > 18 y of age were enrolled based on their willingness to consume both lutein interventions (a supplement pill and a nanoemulsion beverage) for one wk each, to avoid eggs for the study duration and to undergo blood draws four times during the course of the study. Written informed consent was obtained from all subjects before start of the study. The use of human subjects for this study was approved by the University of Massachusetts Lowell Institutional Review Board.
2.2 Study design
The 5 wk sequential study consisted of four phases. All participants started with a 1 wk baseline period of no intervention followed by a one wk period of consuming the lutein supplement (capsule/tablet). This was fol- lowed by a 2 wk washout phase and finally 1 wk of consuming the lutein nanoemulsion beverage. Two different lutein supplements were compared to the nanoemulsion formulation in two separate pilot studies which were done 7 ms apart. Study 1 utilized a 6 mg lutein supplement (Ocuvite® Lutein, Bausch & Lomb, Madison, NJ) whereas study 2 consisted of a multivitamin containing 2 mg of lutein (ICaps MV, Alcon, Fort Worth, TX). Our objective in the 2 mg study was to evaluate bioavailability at a lower concentration and also use a multivitamin matrix containing lutein, as the majority of the older adult population consumes a multivitamin. The lutein supplement source in study 1 was FloraGLO, a highly purified form of lutein obtained by a patented extraction process from marigold flowers [20]. Subjects refrained from eating eggs or other multi- vitamins containing lutein and/or zeaxanthin during the entire study period. Baked food products such as cook- ies, muffins etc. were allowed throughout the study as long as they did not contribute significant amounts of egg yolk. Subjects filled out a 3d diet record once during each phase, which was analyzed using EvaluEat version 1.2 (Copyright© Pearson Education Inc.). As an additional measure of compliance subjects were asked to return empty containers that contained the lute- in supplement and nanoemulsion beverage.
2.3 Blood collection
A 12 h fasting blood draw was done at the end of each phase and blood was collected in red top vacutainers (no additives). Serum was separated by centrifuga- tion at 1500 x g for 12 mins at 4ºC and aliquots were stored at -80º C until analyses.
2.4 Formulation of lutein interventions
Lutein supplements: A 1 wk supply of lutein supplements were dispensed into light protective bottles and handed out to the participants at the end of baseline phase. Participants were given written instructions to consume 1 capsule/tablet per day with a meal and return empty containers at the end of the phase. Lutein nanoemulsions: The nanoemulsion formu- lations were prepared as batches of 200 mL, which were then pooled together to obtain 2L of the nanoemulsion. The procedure for formulating 200 mL of nanoemulsion was as follows: 10 g of food grade soybean oil, 0.67 g of Xangold 15% oil (Cognis, Cincinnati, OH) and 12 drops of natural vitamin E oil (Nature’s Bounty, Bohemia, NY) were weighed in a sterile 500 ml polypropylene beaker. The above specified quantities were used in order to achieve a lutein concentration of 6 mg in 25 mL of the nanoemulsion for study 1. For study 2, in order to achieve a concentration of 2 mg of lutein in 10 mL of the nanoemulsion, 0.45 g of Xangold 15% oil was used. The mixture was then heated and stirred in a water bath at 50 - 60 ºC using a magnetic stirrer until dissolved for about 5 mins. 8 g of Phospholipon 85G (Lipoid LLC, Newark, NJ) was then added and the mixture was heated and stirred again at 60 ºC for 5 mins. Phospholipon 85G was used as it is not toxic to humans and also was shown to pro- duce stable lutein nanoemulsions in pre-clinical studies in our laboratory (unpublished). Natural spring water (Poland Springs, Greenwich, CT) was then added to bring the final volume to 200 mL. The mixture was heated and stirred again for 25 mins at 60 ºC to obtain a homogeneous oil-in-water emulsion. The emulsion was passed through a small volume high shear Microfluidizer® Processor 110S (Microfluid- ics Corporation, Newton, MA) once at 22,000 psi. The Microfluidizer, a patent protected piece of equipment, has a pneumatic intensifier pump, which creates high shear rates by accelerating the sample through micro- channels at a high velocity creating submicron sizes particles [14]. The above steps were repeated until 2 L of nanoemulsion was obtained. Orange juice (Tropicana, Chicago, IL) was used as the mode of delivery of the nanoemulsion because the lutein nanoemulsion formed a homogeneous mixture with the orange juice. The nanoemulsions were added to 10 oz. of orange juice after removal of 25 mL of juice for the 6 mg study and 10 mL of juice for the 2 mg study. The bottles were shaken well and stored at 4 ºC in brown, light protective bags. The participants were given their supply of beverages for the entire week at the end of the washout phase. Participants were given written instructions to consume one 10 oz. bottle of orange juice per day with a meal and to keep the beverages refrigerated.
2.5 Characterization of lutein nanoemulsions
The particle size and polydispersity index of the nanoemulsions were measured using a Zetasizer Nano series (Malvern Instruments Inc. Southborough, MA). This device measures particle size using a process called Dynamic Light Scattering (DLS). DLS (also known as PCS - Photon Correlation Spectroscopy) measures Brownian motion and relates this to the size of the particles. It does this by illuminating particles with a laser and analyzing the intensity fluctuations in the scattered light.
2.6 Serum total and HDL lutein concentration
Serum collected at the end of each phase for each subject was analyzed at the same time for carotenoids. This was done using an Agilent 1100 model (Agilent technologies, Santa Clara, CA) High Performance Liquid Chromatography (HPLC) apparatus with a Diode Array detector, after 100 µL of serum and 200 µL of HDL containing serum were extracted as per the procedure described by Handelman et al. [21]. Serum con- taining HDL was obtained by treatment of 200 µL serum with 200 µL Trace DMA HDL-precipitating reagent (Thermo Electron, Melbourne, Australia) followed by centrifugation at 5000 rpm for 5 mins. The supernatant serum contained HDL while the non-HDL fraction formed a pellet. Concentration of lutein carried on non HDL fraction was calculated as the difference between serum lutein concentration and HDL lutein concentration. The enzyme reagent used for extractions was pre- pared using Cholesterol esterase and Triacylglycerol lipase (Calbiochem, San Diego, CA) that release carotenoids from the carrier lipoproteins. The internal stand- ard used was Tocol [22]. The HPLC column was a 300 mm x 4.6 mm Adsorbosphere HS C18 with 3 µm particle size and 60Aº pore size (Grace Davidson Discover Sciences, Illinois) that was maintained at 18.5ºC during analysis. The solvent system comprised of 40% mobile phase (a mixture of 0.4% ammonium acetate, 50% ace- tonitrile and 50% methanol) and 60% pure acetonitrile for the first 20 mins at 1 mL/min. At 21 mins, 25% iso- propanol and 60% acetonitrile were run for 20 mins, followed by an equilibration period of 20 mins with initial conditions before the next sample.
2.7 Nanoemulsion lutein concentration
Nanoemulsions were extracted to measure the percent recovery of lutein after microfluidization. Aliquots of the lutein nanoemulsion were diluted (1:100) in 0.1M phosphate buffer and extracted in a similar manner as serum. Two mL of 6N KOH was used instead of enzyme reagent and samples were incubated for 30 mins in a 60 ºC water bath followed by extraction with 1:1 hexane-ether [21].
2.8 Serum lipids and lipoprotein cholesterol
Serum lipids and lipoprotein cholesterol concentrations were measured using a Cobas Mira Plus Clinical Chemistry Autoanalyzer (Roche, Branchburg, NJ). Se- rum total cholesterol (TC) [23] concentrations were measured enzymatically using the Infinity Cholesterol Reagent procedure from Thermo Electron (Melbourne, Australia). Serum triglyceride (TG) concentrations [24] were measured enzymatically using Infinity- Triglyceride Reagent procedure from Thermo Electron (Melbourne, Australia). Serum HDL cholesterol was measured directly using Infinity HDL Cholesterol Rea- gent procedure from Thermo Electron (Melbourne, Australia). The concentration of serum LDL cholesterol was calculated via the Friedewald equation. The accura- cy and precision of the procedures used for the lipids and lipoprotein cholesterol measurements are main tained by participation in the Lipid Standardization Program of CDC (Atlanta, GA) and the National Heart, Blood and Lung Institute (NHLBI) (Bethesda, MD).
2.9 Statistical analyses
Sigma stat version 3.1 (Jandel Scientific, San Rafael, CA), a SPSS statistical software package was used for all data analyses [25]. All serum concentrations are ex- pressed as mean + SEM and statistical significance was set at P < 0.05. Percent changes and any significant effect of lutein interventions were compared to baseline unless stated differently. Differences between the 4 phases were determined by repeated measures One-way ANOVA. When differences were observed, a Tukey test was used. Pearson Product Moment Correlation was performed to obtain associations between all the variables measured.
3 Results
3.1 Subjects
Nine participants completed the 6 mg lutein study 1 while 14 participants completed the 2 mg lutein study 2. The age range of the participants was 25 to 65 y with only one being over 60 y. Data from 3 participants in study 2 were excluded for the following reasons. One participant consumed more than 3 eggs at baseline, one could only complete 5 ds of both the lutein interventions instead of 7 ds and the third participant did not normally consume orange juice hence lutein nanoemulsion was added to apple juice for this participant. However, during the course of the study creaming effect occurred in the apple juice containing the nanoemulsion unlike the orange juice. This indicated that the nanoemulsion was unstable in apple juice and thus may not have had the same biological effect as the nanoemulsion in orange juice.
3.2 Diet data
Diet data showed there were no significant differences in mean macronutrient intake across the four phases (data not shown). Amongst the micronutrients, vitamin B6 intake decreased significantly during the lutein supplement and nanoemulsion phases. This was accompanied by a simultaneous decrease in niacin during the nanoemulsion phase but not the supplement phase as one lutein containing multivitamin tablet had 2.5 mg niacin. Although the 7DDR analysis program did not calculate carotenoid intake, the diet records were reviewed to evaluate lutein and zeaxanthin rich foods consumed during the four phases. Spinach, broccoli and corn were the only other foods frequently con- sumed during the study that had substantial amounts of lutein and zeaxanthin [26]. The mean intakes of these foods were not significantly different between the four phases (data not shown).
3.3 Particle size of lutein nanoemulsions
The average particle sizes (Z-average size) of the 6 mg and 2 mg lutein nanoemulsions used in studies 1 and 2, respectively, were 155 nm and 157 nm respectively (Figure 1a). Particle sizes of the nanoemulsions were stable for a period of up to 6 months when stored at 4º C with a polydispersity index (PDI), a width parameter, of less than 0.2 (Figure 1b). Z-average measurements are unreliable for broader measurements where the PDI is over 0.5. Particle size analysis of lutein microemulsion, be- fore microfluidization process, was performed and showed a non-homogeneous mixture with Z-average of about 3 microns and PDI of 1.000 (Figure 1c).The lute- in microemulsion was also highly unstable as creaming was observed in 24 h and hence was not used as an intervention for the study. Particle size graphs of the 6 mg lutein nanoemulsion are shown as an example in Figure 1.
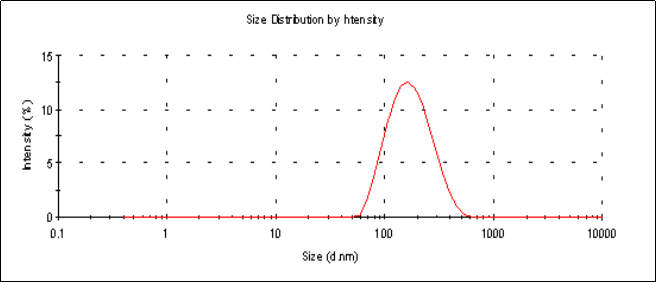
Figure 1.a Particle size graph of the 6 mg lutein nanoemulsion measured using dynamic laser light scattering (DLS) at the start of the study showing an average particle size of 155 nm with a PDI=0.157.
![]()

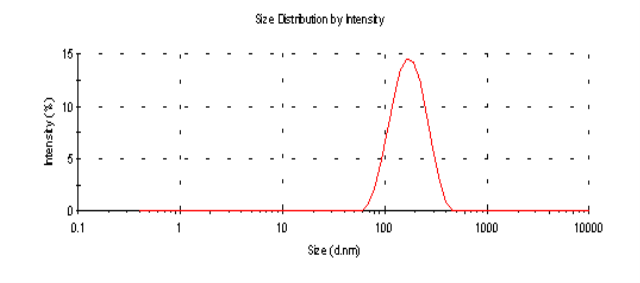
Figure 1.b Particle size graph of the 6 mg lutein nanoemulsion measured using dynamic laser light scattering (DLS) 6 months post study period showing an average particle size of 153.2 nm with a PDI= 0.143.
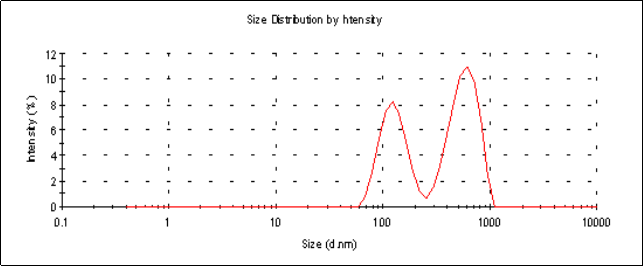
Figure 1.c Particle size graph of a lutein microemulsion (lutein formulation pre-microfluidization) measured using dynamic laser light scattering (DLS) showing an average particle size of 3449 nm with a PDI=1.000. The presence of multiple peaks indicates a non-homogeneous mixture.
3.4 Concentration of lutein and zeaxanthin in the nanoemulsion
In study 1, the actual concentration of lutein in the 6 mg nanoemulsion (n = 6) was 218 µg/mL, which corresponds to 5.45 mg per 25 mL of the nanoemulsion. The supplement used for this study had 6 mg of lutein per capsule. Thus, lutein concentration in the 6 mg nanoemulsion was 10% lower than lutein in the supplement. Unexpectedly, in study 2, the actual concentration of lutein in the 2 mg nanoemulsion (n = 4) was 100 µg/mL, which corresponds to 1 mg per 10 mL of nanoemulsion. The supplement used for this study had 6.67 mg lutein in one serving (4 tablets) and participants consumed 1 tablet per day, which is equivalent to 1.7 mg lutein per day. Thus, lutein concentration in the 2 mg nanoemulsion was 40% lower than lutein in the supplement. The zeaxanthin levels in the 6 mg supplement of study 1 were not reported in the product information sheet while the 2 mg supplement of study 2 reported 3.33 mg in 4 tablets i.e. 833 µg zeaxanthin per tablet. The 6 mg nanoemulsion used in study 1 had 422 µg zeaxanthin per 10 oz. of orange juice while the 2 mg.
![]()
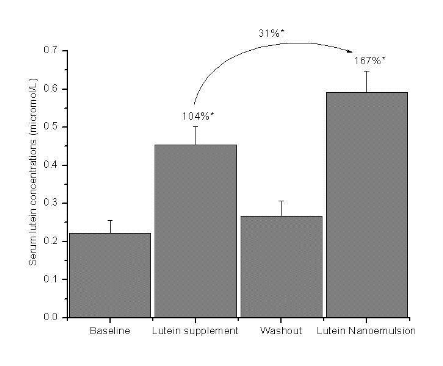
*P < 0.001 using One-way RM ANOVA followed by Tukey test
Figure 2.a Mean + SEM serum lutein concentrations (µmol /L) at baseline, lutein supplement (6 mg/d), washout and lutein nanoemulsion (6 mg/d) phases in study 1 with percent differences between baseline-lutein supplement, baseline lutein nanoemulsion and lutein supplement nanoemulsion phases. n = 9.
*P < 0.001, ** P < 0.050 using One-way RM ANOVA followed by Tukey test
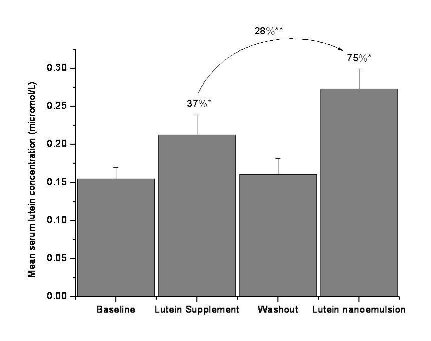
Figure 2.b Mean + SEM serum lutein concentrations (µmol/L) at baseline, lutein supplement (2 mg/d), washout and lutein nanoemulsion (2 mg/d) phases in study 2 with percent differences between lutein supplement-baseline, lutein nanoemulsion-baseline and lutein supplement-nanoemulsion phases. n = 11
![]()
3.5 Serum lutein concentration
Nanoemulsion used in study 2 had approximately 18µg of zeaxanthin. In study 1, mean serum lutein concen- tration increased by 104% (P < 0.001) and 167% (P < 0.001) after one wk of consuming 6 mg lutein per day of supplement and nanoemulsion, respectively, compared to baseline (Figure 2a). There was a 31% (P < 0.05) increase in serum lutein concentration during the nanoemulsion phase compared to the supplement phase. In study 2, mean serum lutein concentration increased by 37% (P < 0.05) and 75% (P < 0.001) after one wk of consuming 2 mg lutein per day of the sup- plement and nanoemulsion, respectively, compared to baseline (Figure 2b).There was a 28% (P < 0.05) in- crease in serum lutein concentration during the nanoemulsion phase compared to the supplement phase. Individual responses of serum lutein concentrations to both the interventions are shown in Table 1. Three participants in the 2 mg study and one in the 6 mg study showed slightly lower (< 10%) serum lutein response to nanoemulsion compared to the supplement. However, the mean serum lutein concentration of these 4 participants during the supplement phase (0.338 + 0.095µmol/L) was not significantly greater than during the nanoemulsion phase (0.307 + 0.091 µmol/L). Participant AE missed one serving of the lutein nanoemulsion.
3.6 Serum zeaxanthin, α-carotene and β-carotene concentrations
Mean serum zeaxanthin concentration increased by 66% (P < 0.001) and 83% (P < 0.001) after the 6 mg lutein supplement and nanoemulsion phases, respectively, in study 1 compared to baseline. In study 2, an in- crease of 46% (P < 0.001) and 19% was observed in mean serum zeaxanthin concentration after the 2 mg lutein supplement and nanoemulsion phases respective- ly. In study 1 with 6 mg lutein, mean serum β-carotene concentration was lowest during the lutein supplement phase (Table 2) with a 30% (P < 0.05) increase after the nanoemulsion phase compared to baseline. Mean serum α-carotene concentrations did not change during any of the phases in both the studies.
Table 1. Individual serum lutein concentrations (µmol/L) at baseline, lutein supplement and lutein nanoemulsion phases of study participants from both study 1 (6 mg lutein) and study 2 (2 mg lutein).
|
Participants |
Sex |
Baseline |
Lutein sup- plement (6mg/d) |
Lutein Nanoemulsion (6 mg/d) |
Baseline |
Lutein sup- plement (2 mg/d) |
Lutein Nanoemulsion (2 mg/d) |
|
RV |
F |
0.224 |
0.327 |
0.561 |
0.275 |
0.332 |
0.404 |
|
LK |
F |
0.181 |
0.386 |
0.616 |
0.124 |
0.199 |
0.339 |
|
TW |
M |
0.212 |
0.345 |
0.537 |
0.159 |
0.235 |
0.302 |
|
RN |
M |
0.080 |
0.621 |
0.733 |
- |
- |
- |
|
SRI |
M |
0.185 |
0.279 |
0.556 |
0.119 |
0.092 |
0.162 |
|
JX |
M |
0.290 |
0.592 |
0.555 |
- |
- |
- |
|
NP |
M |
0.250 |
0.390 |
0.491 |
- |
- |
- |
|
RL |
M |
0.441 |
0.694 |
0.937 |
- |
- |
- |
|
AE |
M |
0.136 |
0.440 |
0.331 |
- |
- |
- |
|
RB |
F |
- |
- |
- |
0.164 |
0.231 |
0.354 |
|
KD |
F |
- |
- |
- |
0.076 |
0.130 |
0.140 |
|
SK |
F |
- |
- |
- |
0.152 |
0.297 |
0.208 |
|
RR |
M |
- |
- |
- |
0.200 |
0.253 |
0.287 |
|
DAS |
M |
- |
- |
- |
0.156 |
0.121 |
0.191 |
|
KG |
F |
- |
- |
- |
0.166 |
0.332 |
0.323 |
|
SE |
F |
- |
- |
- |
0.120 |
0.124 |
0.287 |
Mean serum lutein concentration after baseline and washout phases were the same.
3.7 HDL and non-HDL distribution of lutein
The distribution of lutein between the HDL and non HDL fractions in serum did not change during the supplement and nanoemulsion interventions in both studies 1 and 2 containing 6 mg and 2 mg lutein, respectively (Table 3). In study 1, mean HDL lutein concentration increased by 87% (P < 0.001) and 137% (P < 0.001) after the 6 mg supplement and nanoemulsion phases, respectively, compared to baseline while mean non HDL lute in concentrations increased by 126% (P < 0.050) and 206% (P < 0.001), respectively. In study 2, mean HDL-lutein concentration increased by 40% and 66% (P < 0.050) after the 2 mg supplement and nanoemulsion phases, respectively, compared to baseline while mean non HDL lutein concentration increased by 31% and 86% (P < 0.050) respectively. Approximately 40–50% of zeaxanthin and 12-15% of α-carotene and β-carotene were carried on HDL during all four phases (data not shown).
Table 2. Mean + SEM concentrations in µmol/L of serum zeaxanthin, α-carotene and β-carotene at base line, lutein supplement, washout and lutein nanoemulsion phases in both study 1 (6 mg lutein) and study 2 (2 mg lutein).
|
|
Baseline |
Lutein supple- ment |
Washout |
Lutein nanoemulsion |
|
Study 1 6 mg study (n = 9) Serum zeaxanthin |
0.062 + 0.007a |
0.103 + 0.010b |
0.064 + 0.008a |
0.115 + 0.011b |
|
Serum α-carotene |
0.127 + 0.037 |
0.115 + 0.028 |
0.162 + 0.033 |
0.177 + 0.040 |
|
Serum β-carotene |
0.323 + 0.092a,b |
0.308 + 0.072a |
0.363 + 0.080a,b |
0.401 + 0.090b |
|
Study 2 2 mg study (n = 11) Serum zeaxanthin |
0.060 + 0.006a |
0.087 + 0.010b |
0.063 + 0.006a |
0.071 + 0.008a.b |
|
Serum α-carotene |
0.089 + 0.010 |
0.097 + 0.023 |
0.077 + 0.021 |
0.088 + 0.026 |
|
Serum β-carotene |
0.252 + 0.048 |
0.268 + 0.065 |
0.260 + 0.069 |
0.277 + 0.058 |
Means not sharing a common superscript are significantly different at P < 0.05 using One-way RM ANOVA followed by Tukey test.
Table 3. Mean + SEM serum lutein concentrations and HDL-lutein concentrations in µmol/L with mean percentage of lutein carried on HDL at baseline, lutein supplement, washout and lutein nanoemulsion phas- es in both study 1 (6 mg lutein) and study 2 (2 mg lutein).
|
|
Baseline |
Lutein supple- ment |
Washout |
Lutein nanoemul- sion |
|
Study 1 6 mg study (n = 9) Serum total lutein |
0.222 + 0.034a |
0.453 + 0.049b |
0.267 + 0.040a |
0.591 + 0.056c |
|
Serum HDL-lutein |
0.127 + 0.025a |
0.238 + 0.035b |
0.160 + 0.038a |
0.301 + 0.036c |
|
Percentage lutein carried on HDL |
54.0 + 4.6 |
53.6 + 5.9 |
56.4 + 4.1 |
50.9 + 2.9 |
|
Study 2 2 mg study (n=11) Serum total lutein |
0.155 + 0.015 |
0.213 + 0.026 |
0.161 + 0.021 |
0.273 + 0.026 |
|
Serum HDL-lutein |
0.092 + 0.018a |
0.129 + 0.019a,b |
0.091 + 0.010a |
0.153 + 0.016b |
|
Percentage lutein carried on HDL |
56.9 + 5.7 |
60.5 + 4.3 |
57.8 + 2.8 |
56.4 + 3.6 |
Means not sharing a common superscript are significantly different at P < 0.05 using One-way RM ANOVA followed by Tukey test.
Table 4. Mean + SEM concentrations of serum total cholesterol, HDL cholesterol, LDL cholesterol and triglycerides in mmol/L at baseline, lutein supplement, washout and lutein nanoemulsion phases in both study 1 (6 mg lutein) and study 2 (2 mg lutein).
|
|
Baseline |
Lutein supplement |
Washout |
Lutein nanoemul- sion |
|
Study 1 6 mg study (n = 9) Total cholesterol |
4.34 + 0.17 |
4.52 + 0.25 |
4.58 + 0.31 |
4.33 + 0.33 |
|
HDL cholesterol |
1.05 + 0.09 |
1.04 + 0.08 |
1.08 + 0.06 |
1.03 + 0.06 |
|
LDL cholesterol |
2.60 + 0.16 |
2.35 + 0.26 |
2.86 + 0.22 |
2.63 + 0.20 |
|
Triglyceride |
1.53 + 0.27 |
2.48 + 0.96 |
1.39 + 0.25 |
1.45 + 0.37 |
|
Study 2 2 mg study (n=11) Total cholesterol |
4.52 + 0.28 |
4.61 + 0.30 |
4.46 + 0.26 |
4.41 + 0.31 |
|
HDL cholesterol |
1.18 + 0.09 |
1.24 + 0.10 |
1.20 + 0.08 |
1.16 + 0.10 |
|
LDL cholesterol |
2.66 + 0.27 |
2.81 + 0.24 |
2.88 + 0.24 |
2.73 + 0.26 |
|
Triglyceride |
1.48 + 0.27a |
1.21 + 0.20a,b |
0.85 + 0.10b |
1.16 + 0.17a,b |
Means not sharing a common superscript are significantly different at P < 0.050 using One-way RM ANOVA followed by Tukey test.
3.8 Serum lipids and lipoprotein cholesterol
In study 1, mean serum TC, HDL cholesterol, LDL cholesterol and TG concentrations did not change during any of the phases (Table 4). In study 2, a 74 % (P < 0.05) decrease was observed in serum TG concentration after the washout phase compared to baseline, which may have occurred as some participants were not fasting during the baseline blood draw. No change was ob- served in serum TC, HDL and LDL cholesterol concen trations (Table 4). In study 1, serum lutein concentration was positively associated (P < 0.050) with serum HDL cholesterol concentration at the baseline (r = 0.843), lutein supple- ment (r = 0.757), washout (r = 0.894) and lutein nanoemulsion (r = 0.949) phases. . In study 2, serum lutein concentration was associated (P < 0.050) with serum HDL only at baseline (r = 0.784) and washout (r= 0.627) phases.
4 Discussion
A novel lutein nanoemulsion delivery system was formulated using Microfluidizer® Processor and bioa- vailability was determined in a pilot-scale clinical study. Nanoemulsion delivery systems have previously been shown to improve bioavailability of nutrients such as deltatocopherol, calcium and the vitamin like coenzyme Q-10 molecule [14, 27, 28]. Preclinical studies have shown increased anti-cancer activity of drugs such as tamoxifen and dacarbazine, enhanced anti inflammatory property of aspirin, improved bioactivity of insulin when delivered as nanoemulsions [17-19, 29]. Paclitaxel nanoformulation showed increased bioavailability, efficacy and reduced toxicity in a clinical study [30]. Consumption of 6 mg/d and 2 mg/d of lutein nanoemulsions in studies 1 and 2, respectively, increased serum lutein concentrations by 28% (P < 0.05) and 31% (P < 0.05) respectively compared to consumption of similar doses of its nonnano supplement counterparts. It is noteworthy that lutein nanoemulsions had greater bioavailability even though the actual lutein concentration in the 6 mg and 2 mg nanoemulsions were 10% and 40% lower compared to the lutein supplement. The significantly greater bioavailability of lutein nanoemulsions could have two explanations: (i) nano sized lutein particles and (ii) micelle like matrix of the nano particles. The lutein nanoparticles have a greater probability of being absorbed as they have a greater surface to volume ratio as opposed to lutein in a pill form [31, 32]. The 6 mg lutein supplement pill in the present study was in a powder, not oil form. The oil and surfactant matrix which carries lutein in a nanoemulsion resembles a micellar structure and makes lutein, a normally hydrophobic molecule, water dispersible. During digestion, carotenoids and other hydrophobic molecules are solubilized into mixed micelles for absorption by intestinal cells [32]. It remains a spec- ulation whether orally consumed lutein nanoemulsions would reassemble into mixed micelles along with the ingested foods/carotenoids in the intestinal lumen or whether the nano lutein particles have the ability to get directly absorbed by the intestinal mucosal cells as they already have a micelle-resembling structure. The 167% (P < 0.001) increase in serum lutein concentration after the 6 mg nanoemulsion phase was also greater than the 82% (P < 0.001) increase observed in a study by Chung et al. after consumption of 6 mg lutein/lutein ester supplement for a 10 d period [8]. Serum lutein response was 207% when 8 mg utein was consumed with 36 g of fat while it was 88% with 3 g of fat [33]. In the present study, the 6 mg lutein nanoemulsion contained only 1.25 g of fat and 1 g of phospholipid (85% phosphati- dylcholine) per serving while the 2 mg lutein nanoemulsion contained 0.5 g of fat and 0.4 g of phospholipid. The lutein interventions were not consumed with any additional fat other than that present in the meal with which they were consumed. The lutein supplements also contained 100% of daily recommended allowance for vitamin C so did the lutein nanoemulsions as they were added to orange juice, which has been reported to enhance lutein absorption [34]. Among the four participants who took part in both 6 mg and 2 mg studies, one participant had a 25% greater increase in serum lutein concentration with 2 mg lutein nanoemulsion compared to even the 6 mg lutein supplement. This indicates that a nanoemulsion delivery system may be effective in individuals who do not re- spond to even high dosage of supplements. Pharmaco logical doses of carotenoids are less effectively absorbed as opposed to physiological doses as carotenoid absorption depends on the amount of mixed micelles produced, which in turn depends on dietary triglycerides [32]. The fact the lutein nanoemulsions were signifi- cantly more bioavailable even at 10-40% lower doses than supplements is indicative that nanoemulsions could increase bioavailability even at physiological doses. A 1 wk intervention period was sufficient to study serum responses to increased dietary lutein consumption. Molldrem et al [35] also observed increases in serum lutein concentration after one week of feeding a lutein supplement and lutein containing yellow carrots. The fact that serum lutein concentrations decreased to base- line concentration during the 2 wk washout phase is also consistent with past lutein intervention studies [8,35]. In study 1 containing 6 mg of lutein, the supplement was not reported to have any zeaxanthin while the nanoemulsion had about 422 µg zeaxanthin/ serving but significant increases were observed after consumption of both interventions compared to baseline. A greater concentration of zeaxanthin (833 µg/serving) in the 2 mg supplement compared to the 2 mg nanoemulsion (18µg/serving) may have caused greater increases in serum zeaxanthin after the supplement compared to the nanoemulsion phase. Results from a randomized, blind- ed, cross-over, lutein intervention study using yellow carrots and lutein supplements, showed a decrease in serum β-carotene concentration with simultaneous in- take of lutein supplements but not with intake of lutein containing yellow carrots [35]. The nanoemulsion inter- vention also had a similar effect on serum β-carotene concentration like the yellow carrots in the above mentioned study. Lutein and β-carotene are known to compete with each other for incorporation into chylomicrons; lutein supplemented in a purified form with β- carotene lowers β-carotene chylomicron response [36]. Lutein delivered as a nanoemulsion had an advantage as it did not affect β-carotene absorption. Lutein supple- mentation of up to 6 mg/d, either as a supplement or a nanoemulsion, did not change lipoprotein distribution of carotenoids. HDL carried 50-60% of the serum lutein and zeaxanthin and only 12-15% of α-carotene and β- carotene. The particle size of the lutein nanoemulsion (150 nm) was greater than the particle size of nanoformula- tions of other compounds like ASF, deltatocopherol and aspirin previously studied in our laboratory by microfluidization process, which were all less than 100 nm [14, 15, 19]. The reason for the greater than 100 nm particle size of the lutein nanoemulsion may be the type of surfactant used in the formulation. The nanoformulations mentioned earlier were prepared using 10% Poly- sorbate 80 whereas the lutein formulation was prepared using 4% Phospholipon 85G, a phosphatidylcholine enriched unsaturated phospholipid. The carbon number of the alkyl group in the phosphatidylcholine has a strong correlation with diameter of the particles in the emulsion; a longer carbon chain causes an increase in particle size [37, 38]. The studies that used polysorbate 80 were all pre-clinical studies or cell culture studies. For human consumption, however, FDA has determined that no more than 360 mg of polysorbate 80 can be ingested per day as an emulsifier for edible fats and oils [39]. Since the present study was done in humans, and concentration of surfactant in the lutein nanoemulsions was 1g per serving, polysorbate 80 was not a preferred surfactant. The greater than 100 nm particle size of the lutein nanoemulsion may also be due to the soybean oil in addition to the phospholipid emulsifier. Nanoemulsions composed of oils containing long chain triglycerides (LCT) have considerably larger particle size (120 nm) than nanoemulsions made with low viscosity oils like hexadecane [40]. In conclusion, the present study showed lutein delivered orally as nanoemulsions have greater bioavailability than lutein in a supplement pill form. Also, con- sumption of up to 6 mg of lutein as a nanoemulsion did not affect absorption of hydrocarbon carotenoids, α- carotene and β-carotene, from the diet. Lutein as a nanoemulsion may not require presence of fat for absorption as it is carried in an oil phospholipid matrix, however this hypothesis would need further investigation. The preliminary findings from the present study could be used to design larger, more controlled, clinical studies to study lutein nanoemulsion bioavailability and efficacy.
Acknowledgements
The authors thank the graduate students and staff at the Center for Health and Disease Research at UMass, Lowell who volunteered to participate in the study; Donna Rogers and Beth Halaby, UMass Lowell for per- forming the blood draws; Rosanna Brackett for coordinating with study volunteers and entering diet records; Candice Gendron for entering diet data; Elizabeth F. Goodrow Kotyla for assistance in conducting the study; Maureen Faul for administrative assistance and Micro- fluidics Corp., Newton, MA.
References
Received 10 November, 2009; accepted 6 December, 2009; published online 9 December, 2009.
Copyright: (c) 2009 R. Vishwanathan et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.