Review: Biomedical Applications of Carbon Nanotubes
Mohammad Uzair 1, Muhammad Arshad 1 *, Salman Shabbir Abbasi 1, Abida Arshad 2, Jabar Zaman Khattak 1, Sobia Tabassum 1, Umer Bin Zakaria 1
1 Department of Biological Sciences, International Islamic University, Islamabad, Pakistan.
2 Department of Zoology, PMAS-Arid Agriculture University Rawalpindi, Pakistan.
* Corresponding author. E-mail: m.arshad@iiu.edu.pk
Received: Jul. 27, 2020; Accepted: Oct. 27, 2020; Published: Feb. 22, 2021
Citation: Mohammad Uzair, Muhammad Arshad, Salman Shabbir Abbasi, Abida Arshad, Jabar Zaman Khattak, Sobia Tabassum, and Umer Bin Zakaria, Review: Biomedical Applications of Carbon Nanotubes. Nano Biomed. Eng., 2021, 13(1): 82-93.
DOI: 10.5101/nbe.v13i1.p82-93.
Abstract
Carbon nanotubes are electrically, thermally, and chemically conductive nanoparticles with unique nano-dimensions, unusually strong, and high aspect ratio, which makes them perfect for biomedical applications. Recent researches on carbon nanotubes endow anti-microbial activity against different pathogenic strains of gram-positive (Staphylococcus aureus and S. pyogenes) and gram-negative (Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis), incorporating carbon nanotubes in scaffold for tissue engineering due to their strong characteristics and conducting properties that enhance cell regeneration, and efficacy in drug delivery techniques. Undesirably, the needle-like manifestation of carbon nanotubes and its physical features lead to toxicity problems as respiratory problems, pulmonary toxicity, agglomeration, or cell death. This report aims to describe the possible potentials of carbon nanotubes and their suitability in various aspects of the biomedical field and also highlight their prospects. The antimicrobial activity, applications in tissue engineering, drug delivery, and the toxicology of carbon nanotubes are also discussed here.
Keywords: Carbon nanotubes, Anti-microbial activity, Tissue engineering, Scaffolds, Drug delivery, Toxic effects
Introduction
The two natural crystalline isotopes are Diamond and Graphite. A new isotope, Buckminster fullerene (C60), was discovered in 1985. Besides Diamond, Graphite, and Fullerene, another form of carbon, Carbon nanotubes (CNTs), the strongest and rigid carbon isotopes, were first reported by Iijima [1, 2] in 1991. Carbon nanotubes are well-ordered, all-carbon hollow graphitic nanomaterial, composed of rolled sheets of grapheme built from hexagonal arrangement of sp2-hybridized carbon atoms [1]. CNTs possess peculiar physiochemical properties such that they have high thermal and electrical conductivity, mechanically resistant but very elastic at the same time, having a large specific surface area [3, 4]. CNTs are nanometers in diameter and micrometers in length, exist as either single-walled (SWCNTs), having a diameter of 4.2-2 nm, or multi-walled (MWCNTs) depending upon the number of carbon layers, having a diameter ranging from 2-100 nm [5](Fig. 1). It is reported that mechanical strength and stiffness of carbon nanotubes is 10-100 times higher than that of steel. CNTs have unique features with electrical, mechanical, and optical properties. CNTs retain their self thermally stable up to at 2800ᵒC in a vacuum [6, 7]. Hence, they can be one of the stiffest materials on earth. Thostenson (2007) et al reported different types of carbon nanotubes having varying levels of impurities. CNTs can be obtained by three methods; laser ablation [8], arc discharge method [9] and chemical vapor deposition (CVD) which includes high-pressure carbon monoxide method (HiPco) [10]. Graphene rods are consumed in laser ablation and arc discharge methods as a source of carbon.CVD method uses a metal catalyst and gaseous carbon supply like methane and ethylene, which produces vertically aligned carbon nanotubes as forest on the substrate [11] (Fig. 2). Carbon nanotubes extract from this substrate and fuse them in complex formations. Instead of CNTs forest, carbon nanotubes can be adjusted into sheet and loop into yarn for 3D structures by weaving, knitting or braiding (Fig. 3) [11]. Scientists also have isolated carbon nanoparticles from natural sources i.e. chimney soot [12], kitchen soot [13], etc. Since its discovery by Iijima in 1991 [1], both single and multi-walled carbon nanotubes (SWCNTs and MWCNTs) became a fascinating possibility for biologists. The aforementioned properties of Carbon nanotubes make them entirely appropriate for biological and biomedical applications. Researchers are utilizing CNTs in the biological field from the last two decades and have been exploring their potential in biological and therapeutic applications [16]. CNTs can be a potential candidate for biomedical applications, due to their strong stability, biocompatibility, chemical inertness, high tensile strength, and fast electron transfer kinetics [17, 18]. Carbon nanotubes are currently being consumed for tissue engineering [19], scaffold materials [3], bioelectronics, biosensors [20], drug and vaccine delivery systems [21], antimicrobial activity [22], pharmaceutical, and therapeutic applications [23]. This article intends to brief a review of various research journals deals with carbon nanotubes and their biomedical applications. This article will also discuss the limitations of carbon-based nanotubes and their toxicology.
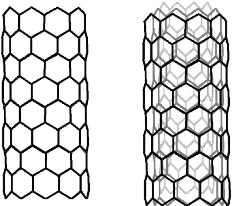
Fig. 1 Single and Multi-walled Carbon nanotubes.
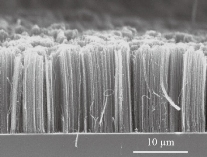
Fig. 2 Cross-sectional SEM micrograph of vertically aligned CNTs-forest [14].
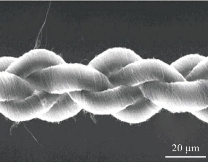
Fig. 3 Scanning electron micrograph of 4-ply carbon nanotube yarn [15].
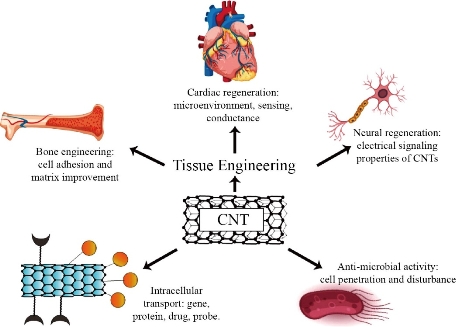
Fig. 4 A schematic representation of prospective CNTs applications in biomedical applications. This figure represents the possible role of CNTs regarding Antimicrobial activity, Drug delivery, and Scaffolding role in tissue regeneration.
Anti-microbial Activity of Carbon-nanostructures
The resistance of microbes against antibiotics is increasing which has led to severe public health problems. The vast majority of the pathogenic microbial strains are resistant against at least one antibiotic. This problem has a lead influence on the researchers to initiate the investigation of novel antimicrobial agents [24]. Researchers and pharmacists are always in search of low cost, less toxic, natural origin and efficient antipathogens. The reconciliation of microbiology and Nanobiotechnology prompts the conceivable development of a new class of antibacterial agents. In recent years, different nanomaterials have been assessed as antimicrobial agents. Currently, researchers analyze the antibacterial activity of carbon-based nanoparticles against chosen gram-positive and gram-negative bacterial strains [13]. Studies demonstrate that carbon nanotubes can assume a strong role in antibacterial action [25]. Kang et al. (2007) first reported the antimicrobial activity of Carbon nanoparticles against Escherichia coli (E.coli). Studies show that single-walled carbon nanotubes (SWCNTs) cause severe cell damage and consequent cell death [22]. CNTs prevent the bacterial cell division and their capability to multiply by penetrating the cell and disturbing its intracellular network. In an examination by Yang et al (2010), the impact of SWCNTs' length on their antimicrobial action was explored. They discovered that longer SWCNTs established stronger antimicrobial action because of their improved aggregation with bacterial cells [26]. The model mechanism of antimicrobial activity of CNTs is still unidentified. The longer CNTs may cause antimicrobial action by covering the bacteria [27]. Carbon nanostructures are reported to cause bacterial cell lysis by physical excoriation and disrupting cell walls and membranes of bacteria ultimately lead to cell death [28]. Some information underpins that accumulation of microbes with CNTs causes’ direct contact between cells and carbon nano-particles which thus lead to cell demise, as shown in Fig. 5 . Moreover, it has been accounted that carbon nanotubes damage the bacteria via phospholipid peroxidation or oxidative stress [29]. CNTs oxidize the bacterial cell which leads towards oxidation resulting in cell death [30]. Owing to their antibiotic potential silver nano-particles have gained much significance for the researchers [31]. Additionally, silver nano-particles are used with the combination of carbon nanotubes to enhance their efficacy. Multi-walled Carbon nanotubes (MWCNTs) are integrated with silver nanoparticles to maximize the anti-bacterial activity and minimize the toxicity of both nanoparticles and absorbance of silver by the body. Ag-MWCNTs show an efficient antimicrobial property and relatively less toxic effect [32]. Arias et al. show that Single-walled Carbon-nanotubes (SWCNTs) with -OH and -COOH functional groups develop more antibacterial action toward both gram-positive and gram-negative species. Functionalized carbon nanotubes form cell-CNTs collections which caused damage to cell walls due to which bacterial DNA releases from the cell. He showed that the same functional groups were attached with MWCNTs but no considerable antibacterial effect was examined [33]. Thomas Varghese et al. (2018) investigated the antimicrobial activity of Carbon nanotubes in well diffusion assay against different gram-positive (Staphylococcus aureus and Streptococcus pyrogenes) and gram-negative (Proteus mirabilis, Klebsiella pneumoniae and Escherichia coli) bacteria. Upon their findings, the better antimicrobial action of CNTs was seen contrary to tetracycline. The zone of inhibition (in cm) by CNT sample was larger than standard antibiotic [12]. Carbon nanotubes exert a potent antimicrobial activity, even in pure form or modified with biocompatible polymers. It can be an active antimicrobial agent and in future perspectives, it will be utilized in pharmaceutical and therapeutic techniques.
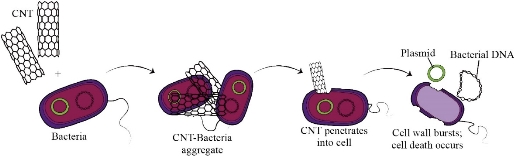
Fig. 5 Supposed mechanism of CNTs Antimicrobial activity. CNTs clustered against bacterial cells and then tear down them by penetration.
Table 1 Zone of inhibition of CNTs against gram-positive and gram-negative microbes [12]
|
Microorganisms |
Zone of inhibition (cm) |
|||
|
Control |
CNTs’ Concentration (µg) |
|||
|
50 |
150 |
250 |
||
|
Escherichia coli |
23 |
_ |
10 |
12 |
|
Proteus mirabilis |
18 |
11 |
16 |
22 |
|
Klebsiella pneumonia |
25 |
10 |
14 |
21 |
|
Streptococcus pyogenes |
24 |
0 |
0 |
15 |
|
Staphylococcus aureus |
25 |
0 |
12 |
21 |
Resistant: <9 mm, intermediate: 15–20 mm, sensitive: ≥20 mm.
Carbon Nanotubes in Scaffold for Tissue Engineering
Tissue engineering is the way to construct new tissue or to repair damaged ones mainly achieved by stem cells, which generate biological tissue resembled the body's native tissue. Tissue engineering requires a biodegradable scaffold that provides an adhesive surface to seeded cells [34]. Tissue engineering is the crossroad of stem cells and biotechnology. The scaffold should be permeable that support tissue development and allow cells to grow into an accurate structure [35]. Constructed scaffold must be in a 3-dimensional structure which can also transport nutrients to cells. It should be electrically conductive that allows cell multiplication and differentiation [36]. Biomedical engineers incorporate nanotechnology and tissue engineering to enhance scaffold properties. The utilization of Carbon nanotubes in the tissue engineering scaffold opens a new way for the researchers. CNTs improve the scaffold properties which enhance tissue engineering techniques [11, 19]. Different nano-materials vary in the properties of the scaffold. Carbon nanotubes have proved their significance in scaffold properties as they are durable and electrically, chemically, and thermally conductive [37]. Fibrous CNTs composition is analogous to cell supporting extracellular matrix, which has shown a remarkable improvement in cell maturation [37]. Cells show better bonding, extension, and proliferation of CNT-based scaffolds [38]. Loose carbon nanotube in suspension and CNTs enclosed in the structure were scrutinized for the in vitro biocompatibility test [39]. Studies proved that loose carbon nanotube suspended in cell culture lessens cell viability. In contrast, cells affixed to carbon nanotube containing scaffold supported better cell growth and general biocompatibility with living cells was demonstrated [40]. In chemistry, CNTs are inactive and do not show any biological action. Hence, CNTs themselves are not biomaterial so they cannot be utilized directly in tissue engineering. To make them biocompatible various surface modifications are required so that they can maintain cell maturation [11].
CNTs in neural regeneration.
Humans are constrained in their capacity to recover nerve cells [41]. Due to this, the treatment of damage and disease of the nervous system is still a challenging issue for biomedical engineers. Several studies were done to reinstate complex neuronal connections in the brain after neuron or brain injury [42]. Nanotechnology offers a promising innovative methodology for the treatment of neural regeneration. Carbon nanotubes may provide an evolutionary path for neuronal tissue engineering as it imitates the tubular forms of microtubules and axons [43, 44]. Also, CNTs are electrically conductive and possess <100nms in length, which shows a close resemblance to nerve fiber. CNTs substrate with certain conductivity encourage neuronal development and also augments the mean area of the neural cell body. Hence, they have the potential to regenerate neurons [45, 46]. Carbon nanotubes can be the favorable substrate on which neurons can nurture and exist. Neural survival, as well as growth, was observed on CNT-based scaffold [45]. Matson et al. (2000) reported that neurites of rat hippocampal neuron grow and extends in all directions on MWCNTs modified with 4-hydroxynonenal (4-HNE) deposited on polyethyleneimine (PEI) layered glass slips, in contrast with un-modified MWCNTs. But branches did not appear in this milieu [47]. Surface charge of CNT is also a significant aspect in neural growth, elaborated branching and neurites length. It has been reported that positively charged MWCNTs allow neurites' enhance growth, elongated neurite length, and further branching in neurites as compared to negatively charged CNTs [48]. The roughness of CNTs and the exposed area play a significant role in neuronal adhesion. Selective serum protein coat, for instance, fibrinogen and apolipoproteins on CNTs also sustain cell adhesion [49]. For neural tissue engineering, required scaffolds must be electrically conductive. This can be achieved by CNTs as they are electrically conductive. CNTs based scaffold for the neural tissue engineering presumes to imitate the electrical properties of nerves and neurons so that they can electrically stimulate neuron cells [3, 44]. Ni et al. (2005) investigate another probability by adding CNTs directly into the neural cell culture medium. Carboxyl functionalized single-wall carbon nanotubes (COOH-SWCNTs), with open ends, were modified with polyethylene glycol (PEG) or poly amino benzene sulphonic acid (PABA). These modified CNTs were then integrated into the cell culture of hippocampal neurons [50]. The results demonstrated that SWCNTs promote neuronal growth, but neurites lessen in number. These functionalized and modified CNTs were also utilized to graft polymers. Studies show that increased length of neurites was observed on polymers grafted with SWCNTs [50]. These results show that CNTs can promote neurites' development at the site of nerve injury, consequently enhancing the neural system in the brain. Outcomes are captivating, as it opens a new approach in neural system regeneration.
CNTs in bone engineering
Bone tissue engineering is still a major challenge for biomedical engineers. To develop a biocompatible scaffold that can enhance the growth and proliferation of osteoblast is the main task [51]. Bone is a major component of the skeletal system and provides structure and support to the body. Bone tissue consists of the fibrous protein collagen, saturated with hydroxyl apatite (HAp), closely resemble calcium phosphate apatite. Hydroxyl apatite is a matrix protein secreted by Osteoblasts, fabricated on the surface of the bone [52]. Osteoblasts are Bone-forming cells and one of the major cellular components of bone along with Osteoclasts (which absorb bone tissue) and osteocytes (mature osteoblast retain bone integrity) [53]. Researchers are focused on studies dealing with biopolymer's applications. Bone grafts are used to heal the complicated fractures of the bone. Artificial bone scaffolds made up of filamentous peptide and polymers are utilized in bone grafting. But these materials have comparatively low efficiency and show sensitivity to the immune system [27, 46, 51]. CNTs can be a promising part of bone regeneration and grafting, due to its strong mechanical strength, flexibility, and low compactness [54, 55]. As aforementioned CNTs are the rigid material founded and by incorporating them in scaffold its properties can be enhanced, which provides compatible milieu to the bone cells. Saffar et al. (2009) cultured osteoblast cells on CNT-based scaffold. The porous shape and mechanical strength of bone were retained due to CNTs based scaffold [56]. Polymethyl methacrylate (PMMA), generally named as Acrylic bone cement, is a common biopolymer used for orthopedic surgeries, bone, and dental filling cement [57]. In contrast to natural bone tissue, PMMA is weaker in mechanical strength. Marrs et al. (2006) observed that mechanical properties and toughness of PMMA increases when CNTs are integrated with them, which improves the implant's durability. The resulting bone has improved resistance, against fatigue and fracture [58]. Aoki et al (2007) observed that Osteoblast cells show strong adhesion to CNTs [59]. Considerably more bone regeneration, better cellular fabrication, and higher osteoconduction occur on CNT based porous 3d scaffold. Cell division and adhesion were remarkably increased on CNTs coated surfaces [60]. The aforementioned properties of CNTs can reinforce and toughen Hydroxyl apatite (HAp), whereas maintaining its biological activity [61]. In contrast to neutral surface CNTs, charged-surface CNTs inhibits the growth and fabrication of osteoblast cells. CNTs modified with chemical functional groups enhance the scaffold's conductive property to promote the development of artificial bone material. Chemically functionalized CNTs make them more biocompatible as well as provoke new characteristics [62]. Osteoblasts show better growth and differentiation on SWCNTs and MWCNTs chemically modified with a carboxyl (-COOH) functional group. Carboxylate ions (COO-) absorb calcium ions (Ca+2) in COOH-CNT composite, which leads to the crystallization of Hap [63]. Hydroxyapatite (HAp) shows nucleation and mineralization on the surface of SWNTs functionalized with phosphates and polyaminobenzene sulfonic acid (PABS) in the solution phase. Improved characterization of HAp occurs when calcium ions (Ca+2) were attracted to CNTs due to negatively charged functional groups [64]. CNT based bone tissue engineering has the potential to be utilized in the future for orthopedic surgeries and to strengthen bone implants.
CNTs in cardiac tissue regeneration
Cardiovascular Diseases (CVDs) cause the highest mortality rate globally. The most pervasive cause of death amongst cardiac patients is Myocardial infarction (cardiac tissue death) [64]. This is due to the lack of cardiac tissues' regeneration capability [65]. Regenerating cardiac tissue can save the lives of many patients suffering from heart diseases. Cardiac tissue engineering can be a potential cure for cardiovascular disease treatment [65]. Carbon nanotubes introduced as scaffolds for cardiac tissue engineering due to their conductive property [66]. Collagen is a natural polymer that was also utilized in the scaffold. Scaffolds with CNTs/collagen composite shows significant electrical conductivity and biocompatibility to formulate cardiac tissues [67]. Anna M et al. prepared scaffold for cardiac tissue engineering based on CNTs/chitosan composite and only of chitosan. The metabolic action of myocardiocytes was considerably boosted in CNTs/chitosan composite than merely chitosan scaffold. CNTs integration also increases the cardiac-specific gene expression profile, responsible for electrical conductivity and muscle movement [67]. Carbon nanotube-based scaffolds provide artificial but feasible micro-milieu for cardiomyocytes regeneration and functionality [66, 68]. Integrating CNTs in scaffolds for cardiac regeneration not only improves electrical conductivity but also enhance cardiomyocytes fabrication, growth, and segregation [68]. CNTs endorse cell-cell adhesion and electrical communication between cells due to which cell generated can exhibit synchronous beating [69]. Scaffolds based on carbon nanotubes, pure or chemically functionalized, can enhance cell adhesion, differentiation, maturation, and proliferation. It promotes and maintains the viability, integrity, arrangement, and biological activity of the cell. CNTs scaffolds also have the ability to defend cells against the pathological activity. Moreover, CNT scaffolds are biologically compatible. Its 3D porous arrangement has the potential to transport of nutrients and metabolic wastes. These properties prove the beneficial role of CNTs to be utilized as a scaffold for effective tissue engineering techniques.
Carbon Nanotubes in the Targeted-drug Delivery System
The drug delivery system is captivating much concentration of pharmaceutics and therapeutics. Now researchers are introducing Nanotechnology in drug delivery processes, for more efficient and effective outcomes. CNTs have been introduced in pharmaceutics and therapeutics for targeted drug delivery, since the 21st century [5, 71]. The peculiar features of CNTs, such as broad surface area, high aspect ratio, and strong stability enables them to utilize in drug-delivery systems. The characteristics of CNTs make them efficient to load drugs in them or to attach drugs with their surfaces, respectively. Due to their distinctive properties, they can be a potent nano-carrier for the deliverance of drug, biomolecule, gene, DNA/RNA, enzymes/proteins to a targeted organ, cell or tissue [20, 72]. CNTs can penetrate cells and pass through the blood-tissue barrier due to their needle-like shape [16]. The needle-like shape of CNTs plays a vital role in drug delivery as it enhances the penetrating property of CNT, without damaging to cell structure. The large surface area of CNTs develops drug loading capacity [73]. The drug can be transported in two ways via loaded in CNT structure or can be appended to the surface of CNT. Lamberti et al. (2015) propose two methods of CNT associated drug delivery [30]. Either both CNTs and drugs penetrate the cell or drug-loaded CNTs affixes with the cell surface and infuse drugs within the cell [16]. MWCNTs and SWCNTs have a different mechanism of cell penetration. SWCNTs are capable of internalized into cells, while MWCNTs are expelled from the internal cell [74]. Cellular uptake of the CNTs can be manipulated by their size. Lengthy SWCNTs remain in the cytoplasm, whereas short SWCNTs penetrate the nucleus [75]. CNTs-Drug complex enters into the cell through endocytosis (cellular uptake) or by diffusion and insertion. Incorporation and penetration of the CNT-Drug complex shows more effective outcomes than the surface attachment of CNTs [76]. These progressions in nanotherapeutic by CNTs-based drug delivery were introduced in cancer treatment and therapy. Antibiotics, antineoplastics and chemotherapeutic agents were coalescing with CNTs for treatment of infection and tumors.
CNTs in anti-cancerous treatment
Despite having importance in cancer treatment, the drawbacks of chemotherapy such as toxicity, insolubility, incapable of selecting cancerous tissue or cell, inability in trouncing multi-drug resistance(MDR), inability to exceed Blood-Tissue barrier are yet a challenge for the researchers [77, 78]. Consequently, there is a need for a drug that is capable of targeting cancerous mass with broad therapeutic properties. Idiosyncratic properties of CNTs offer an opportunity to overcome chemotherapeutic limitations. Since the last decade, researchers have a main focus on CNTs-based cancer therapy [71, 78, 79]. Lamberti et al. (2014) modified CNTs fused with several chemotherapeutic drugs like doxorubicin, paclitaxel, cisplatin, epirubicin, methotrexate, quercetin, etc. In-vivo and in-vitro determination of these Chemotherapeutic-CNTs complex were successful.[80] CNT's ligand identifies cancer receptors. The Chemotherapeutic-CNTs complex bound to antigens expressed over the cancerous surface. In this way, CNTs recognize cancer cells and deliver drugs selectively and efficiently at the targeted point [76]. CNTs can be labeled with fluorescent agents to detect CNTs' passage towards cancer point. Madani et al. (2011) incorporated CNTs and fluorescent Quantum dots, to enhance cancer-targeting capability. Quantum dots image cancerous cells while CNTs utilized in cancer imaging as well as transports drugs [21]. CNTs modified with polyethylene glycol (PEG). PEG-CNT enhance water-solubility, decrease cytotoxicity, and increases biocompatibility. The PEGylated CNTs not only augment blood circulation but also significantly improve cellular uptake of the drug by the cancer cells [21]. Paclitaxel (PTX) is an anti-cancer drug that is less soluble in water. PEG-CNTs were conjoined with paclitaxel (PTX) which restrains tumor growth and increases blood circulation. Increased growth of PEGylated MWCNTs was observed in multi-drug resistance (MDR) [81]. Lobaplatin (LBP) is a chemotherapeutic drug used against Lung cancer. But LBP exhibits an adverse toxic effect on the body's tissues during lung cancer therapy. Yu et al (2018) conjugate Carbon nanotubes chemically functionalized by polyethylene glycol (PEG-CNTs) and LBP to lessen the toxic effect of LBP [82]. Fluorescein isothiocyanate (FITC) was labeled with a targeted drug delivery system (LBP–PEG–CNTs). HepG2 (human liver cancer cell line) cells were treated with LBP–PEG–CNTs to study its inhibitory effect. The FITC-labeled PEG–CNTs reveal targeted cell penetration and show enhance stability, biocompatibility, and controlled pH milieu. LBP–PEG–CNTs suppress HepG2 cell growth [82, 83]. CNTs exhibit effective outcomes in the targeted drug delivery system and anti-cancer drug delivery. CNTs have potential to be the future of cancer therapy as they able to deliver therapeutic agents to the targeted sites. Even they have no potential side effects and can work accurately. Many researches have been done and many are in progress to prove the CNTs as the futuristic agents of drug delivery at specific sites.
Mediated carbon nanotubes for drug delivery
Medical Grade Molecular Rebar (MGMR) ™ is the world's first mediated carbon nanotube produced by BioPact (Nanotechnology-based medical company in Cambridge) [84, 85]. MGMR™ is a breakthrough that is exploiting by biotechnologists, pharmaceutics, and therapeutics. MGMR™ is a potential medicinal product with moderately long length, smaller diameter, broad surface area, and high aspect ratio. These properties let them penetrate cells and cross the tissue-blood-brain barrier [86]. Larger biomolecule (carbohydrates, lipids, proteins, enzymes, antibodies, etc) and smaller biomolecule (drugs, RNA/DNA, monosaccharide, etc) bind covalently or non-covalently to the exterior surface and loaded inside of MWCNTs respectively (Fig. 6). Insulin, bortezomib, and chlorambucil are loaded in MGMR. To target intracellular peptides is challenging in drug delivery but MGMR™ has accomplished this task by penetrating within minutes. Biopact's MGMR™ drug delivery technology successfully transmits cell impermeable peptides across cell membranes causing absolute death of cancerous cells and tissues. MGMR has the capability to controlled release, targeted and transversal delivery of drugs, and to overcome problems like instability or toxicity, intracellular delivery and exceeding the blood-brain barrier [84-86]. Innovative MGMRTM technology allows researchers to securely and efficiently develop new drug delivery mechanisms.
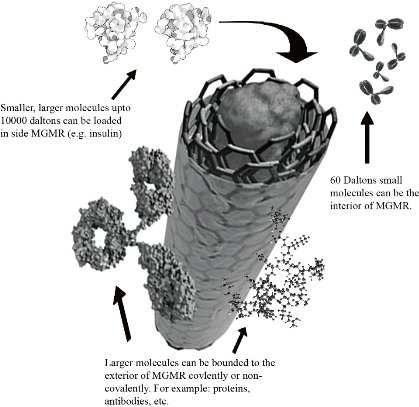
Fig. 6 An illustration of MGMR™: drug loading and binding
Toxicity Problems regarding CNTs
Inimitable characteristics of Carbon nanotubes make them suitable for the biological field but their Asbestos-like structure and needle-like appearance have been raising severe problems concerning their toxic effect. CNTs mostly affect the lungs and respiratory tract [87, 88]. Asbestos is crystal fiber, a well-known hazardous substance. Inhaling Asbestos cause’s lung cancer, scars in lungs and Asbestosis. CNTs almost appear similar to Asbestos in structure [89, 90]. CNTs' internalization in the lungs can cause lung-lining tumor, mesothelioma, and asbestosis-like syndromes [91]. Researchers examine lung fluids of 64 Parisians patients suffering from asthma. They found that every sample has Synthetic CNTs in them [90, 92]. These observations demonstrate that CNTs can affects respiratory system and cause life threatening diseases.
Reducing toxicity effect by functionalization
CNTs toxicity can be manipulated by various factors depending on its morphology, dimensions, chemical, and physical features. Existence of impurities, charge, and agglomeration are also involved in CNTs toxicity. CNTs with impurities cause cell fatality by damaging mitochondria or by oxidative stress[93, 94]. Non-functionalized CNTs also exhibit carcinogenic effects [87]. CNTs toxicity can be reduced by chemical modification and functionalization. Pristine CNTs are hydrophobic but chemical modification biocompatibility and solubility of CNTs can significantly be improved [95]. CNTs can be covalently or non-covalently functionalized which considerably reduces its toxicity (Fig. 7). CNTs modified properly were consumed by B and T lymphocytes and macrophages without disturbing cell feasibility. Functionalized CNTs cause no damage to the cells [96, 97]. Thus, appropriately purification and functionalization of CNTs can lead to the lessening of its toxic effects, enhancing the safety of use making CNTs predominantly potential nanostructures to be utilized in biomedical applications.
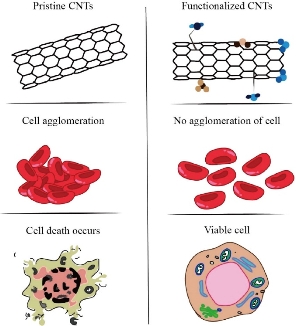
Fig. 7 Effects of Non-functionalized and Functionalized CNTs on cells. Non-functionalized CNTs disrupts cell structure through penetration or cause clustering in cells whereas Functionalized CNTs prevent such problems.
Conclusions and Remarks
Exceptional and peerless properties of Carbon nanotubes makes them very suitable nanostructures for biomedical applications. They are extraordinarily strong, electrically and thermally conductive, with high aspect ratio and of nano-dimensions. Researchers are exploring CNTs' efficacy in the biomedical field since their discovery, two centuries ago. Carbon nanotubes show a promising perspective in various aspects of biomedical applications and researchers are still exploring their innovative possibilities. CNTs are potential pioneers for diagnostics and therapeutics applications. They are not only capable of targeted drug delivery but also exhibit strong antimicrobial activity, owing to unusual long length and needle-like appearance let them penetrate cells. CNTs resembling the needle-like and asbestos-like shape also determine their toxic effect. Conducting properties of CNTs provide an opportunity to researches for utilizing them in tissue engineering. CNTs-based scaffold enhances cell conductance, adhesion, and proliferation. Chemical functionalization of CNTs reduces toxicity and improves their biological activity. Many papers on CNTs are published after their discovery and for biomedical researchers, tissue engineering and anti-cancer therapy are hot topics these days. CNTs provide a cross-road between nanotechnology and medicinal biotechnology. This review emphasizes that more research is needed for innovations in Nanotherapeutic and Nanobiotechnology as the nano-medicine field is rapidly developing and distinguished for accurately delivering pharmaceuticals agents to a targeted site. For this innovative nanoparticles are needed with less toxic effects and more features.
References
Copyright© Mohammad Uzair, Muhammad Arshad, Salman Shabbir Abbasi, Abida Arshad, Jabar Zaman Khattak, Sobia Tabassum, and Umer Bin Zakaria. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.