Research Article
Evaluation of In-vitro Biocompatibility and Antimicrobial activities of Titanium Dioxide (TiO2) Nanoparticles by Hydrothermal Method
Santhanakrishnan Mahalakshmi, Parthasarathy Vijaya*
Department of Nanoscience and Technology, Bharathiar University, Coimbatore - 641 046, India.
* Corresponding authors. E-mail: vijayaparthasarathy@buc.edu.in Tel.: +91 9840868328
Received: Feb. 18, 2020; Accepted: Nov. 16, 2020; Published: Jan. 19, 2021
Citation: Santhanakrishnan Mahalakshmi, Parthasarathy Vijaya, Evaluation of In-vitro Biocompatibility and Antimicrobial activities of Titanium Dioxide (TiO2) Nanoparticles by Hydrothermal Method. Nano Biomed. Eng., 2021, 13(1): 36-43.
DOI: 10.5101/nbe.v13i1.p36-43.
Abstract
Titanium dioxide (TiO2) nanoparticles (NPs) are one of the nanomaterials that have been widely used in cosmetics, pharmaceuticals and biomedical applications which include drug delivery, cancer treatment, biosensors, and genetic engineering. In this study, TiO2 NPs have been synthesized via hydrothermal method. The samples were calcinated at different temperatures such as 450 and 500 °C. The structural, functional group, morphological and elemental composition analyses of the synthesized materials were conducted by various techniques such as X-ray diffraction (XRD), Fourier transform infrared (FTIR) analysis, Raman spectroscopy, field emission scanning electron microscopy (FESEM) and elemental dispersive X-ray (EDX) analysis. Various concentrations (25, 50, 75 and 100 μg/mL) of synthesized TiO2 NPs were evaluated for their antimicrobial activity against the gram-positive and gram-negative bacteria. The maximum zone of inhibition was observed in the synthesized TiO2 NPs (100 μg/mL) against Staphylococcus aureus (15 mm), Bacillus Subtilis (16 mm), Escherichia coli (18 mm) and Pseudomonas aeruginosa (17 mm), and the results indicated that the antimicrobial activities of the TiO2 NPs were tested against pathogenic bacteria. Overall, the results indicated that TiO2 nanoparticles could elicit the viability of lymphocytes at all investigated concentrations (50, 100, 250 and 500 μg/mL). Hence, we concluded that the in-vitro hemolytic and antibacterial activities of TiO2 NPs, indicated the good potential biomedical applications.
Keywords: Titanium dioxide nanoparticles, Hydrothermal method, Antibacterial activity, Agar well diffusion method, Hemolytic activity
Introduction
Nanotechnology is the control and designing of new materials at the nanoscale level with a length of at least one dimension <100 nm [1]. With the improvement and enlarging research interest of nanotechnology in nanoparticles and nanomedicine have prompted a huge potential for novel methods for rapid disease diagnosis, treatment and enhanced quality of life [2]. Nanoparticles have possessed unique size- dependent physical (optical, catalytic), chemical and electrochemical properties, which give helpful attributes for biomedical applications for faster and more reliable methods to assess their safety [3, 4]. It can be classified into carbon based materials (e.g. fullerenes, carbon nanotubes), inorganic NPs such as metal (e.g. silver, palladium and gold), and metal oxides (e.g. aluminum oxide, titanium dioxide, zinc oxide, copper oxide, silicon oxide) and quantum dots [5]. Titanium dioxide, also known as titania (TiO2) is a very useful semiconducting transition metal oxide nanoparticle [6]. TiO2-NPs account for over 70% of the total production volume of nanoparticles around the world [7]. TiO2 is the promising material as semiconductor and exhibits unique characteristics such as easy handling, non-toxicity and low cost [8]. It has four different crystalline polymorphs, namely rutile (tetragonal, a=b=4.59Å, c=2.95Å), anatase (tetragonal, a=b=3.78Å, c=9.50Å), brookite (rhombohedral, a=5.43Å, b=9.16Å, c=5.13Å) and TiO2 (B) [monoclinic, a = 12.16 Å, b = 3.74 Å, c = 6.51 Å] [9] and its band gap values of anatase (3.2 eV), rutile (3.02 eV), and brookite (2.96 eV) phases, respectively [10]. TiO2 nanoparticles is a well-researched material due to excellent dielectric, gas-sensitive, physical, optical, electrical properties, high chemical stability, biocompatibility with great optical transparency, refractive index and wide band gap energy [11]. Titanium dioxide nanoparticles (TiO2 NPs) are used for wide range of applications, such as catalysis, white pigment for paints, cosmetics, food colorants, pharmaceuticals and biomedical fields [12]. Its photocatalytic properties have been utilized in various environmental applications such as air purification and waste water treatment [13]. Recently, TiO2 nanostructures with various morphologies prepared by various methods, such as, nanocrystals, nanorods, nanowires, nanotubes and mesoporous structures [14]. A significant area of research in nanotechnology deals with the synthesis of titanium dioxide nanoparticles (TiO2 NPs) of different chemical compositions, dimensions and controlled monodispersity [15]. The preparation of ultrafine titanium dioxide nanoparticles has been investigated using various methods such as hydrothermal, solvothermal, chemical co- precipitation, chemical vapour deposition (CVD), sol-gel technique, sputtering, hydrolysis and micro emulsion method [16]. Hydrothermal method is one of the most common and effective synthetic routes to fabricate the TiO2 NPs due to its simplicity and high yield [17]. In this method is based on the ability of water and aqueous solutions to dilute at high temperature (500 °C) and pressure (10 - 80 MPa, sometimes up to 300 MPa) [18]. Advantages of the hydrothermal synthetic route include high purity, ease of large scale production, high-crystallized powders with narrow grain size-distribution, environmental friendliness and less hazardous [19]. Furthermore, the disadvantages of hydrothermal methods include high cost of equipment and long synthesis duration [20]. In spite of the antimicrobial activity of TiO2 nanoparticles was demonstrated by the present concern for the experts due to the development of microbial resistance against metal ions, antibiotics and enhancement of resistant [21]. The Quantification of hemolytic activities of a nanoparticle is considered as one of the most fundamental tests in determining the safety of a blood contacting biomaterial. In this method, in-vitro studies of particle induced hemolysis evaluate the percentage of hemolysis by spectrophotometriclly method to detecting plasma-free hemoglobin derivatives after incubating the particles with blood and then separating undamaged cells by centrifugation [22]. In the present work, we have synthesized TiO2 nanoparticles via hydrothermal method and characterized by Powder X-ray Diffraction, Raman Spectroscopy, Fourier Transform Infrared Spectroscopy, Field Emission Scanning Electron Microscope, Energy Dispersive Spectroscopy, UV-visible spectroscopy and performing the antimicrobial and hemolytic activity of titanium dioxide nanoparticles [23].
Experimental
Materials
Different chemicals and reagents used during this experiment including titanium tetra isopropoxide [Ti (OCH (CH3)2] 4, isopropanol (CH3CHOHCH3), ethanol (C2H5OH), dimethyl sulfoxide (DMSO) (CH3)2SO, Muller Hinton agar were purchased from Sigma Aldrich Pvt Ltd., India. All glassware was washed with sterile distilled water and dried in hot air oven before use. The distilled water was used as solvent to prepare the solutions.
Collection of bacterial pathogens
Gram positive bacteria such as Bacillus subtilis (MTCC 1305) and Staphylococcus aureus (MTCC-3160), Gram negative bacteria (Escherichia coli (MTCC-1677) and Pseudomonas aeruginosa (MTCC-4030) were procured from Microbial Type Culture Collection (MTCC), India. All the microbial cultures were stored and maintained at 4 °C.
Synthesis of titanium dioxide nanoparticles
Titanium dioxide nanoparticles were prepared via hydrothermal method using the titanium tetra isopropoxide, isopropanol and distilled water as the starting materials. Titanium tetra isopropoxide (TTIP) and isopropanol at 1 : 4 molar ratio was added to 10 mL distilled water to get white color solution formed, then stirred for 1 h. Clear solution formed after that, the solution was transferred into a Teflon vessel and placed in hot air oven and set temperature at 180oc for 12 h. After 12 h, the autoclave was cooled down to room temperature, then removed the supernatant, washed the precipitates several times with distilled water and ethanol. TiO2 nanoparticles were dried at 80oC at 4 h. Finally, the powder was annealed at different temperature for 450 and 5000C for 4 h.
Characterization of TiO2 nanoparticles
The XRD pattern of TiO2 nanoparticles was obtained by an X-ray diffractometer (XRD, Rigaku Ultima IV) with CuKβ radiation at 25 ºC and the structural assignments were made with reference to the JCPDS powder diffraction files. Functional group analysis of the compound was examined by using Fourier Transform infrared spectroscopy (BRUKER, TENSOR 27, INDIA). Chemical structure of the material was analyzed by Raman Spectroscopy (HORIBA, LabRAM HR). The surface morphologies of TiO2 samples were studied using FE–SEM FEI Quanta 250. Chemical composition of the sample was analysis using energy dispersive spectrum (BRUKER INDIA). UV-visible absorption spectra of TiO2 nanoparticles were recorded using a JASCO V-650 Spectrophotometer.
Antimicrobial activity of TiO2 nanoparticles
The in-vitro antimicrobial activities of the TiO2 nanoparticles were evaluated by agar well diffusion method against Gram + ve bacteria (S. aureus and B. subtilis) and gram –ve bacteria (P. aeruginosa and E. coli). In Brief, 20 mL of Muller Hinton Agar (MHA) was poured into petri dishes and allowed to solidify. Then, 6 mm thick sterile discs were placed appropriately on petridishes. Finally, different concentrations of TiO2 NPs (25, 50, 75 and 100 µg/mL) were dissolved in DMSO and loaded on each disc. The standard drug streptomycin (10 µg) was used as a positive control to determine the sensitivity of each microbial species tested. All the plates were incubated at 37 °C for 24 hours and the respective inhibition zones were measured in millimeters range. The results (mean value n=4) were recorded by measuring the zones of growth inhibition surrounding the disc.
Hemolytic activity of Titanium dioxide nanoparticles
Isolation of erythrocytes from human blood
Heparinized fresh human blood was collected from healthy volunteer. The collected blood sample was then mixed with 0.2 M phosphate buffered saline (PBS) (pH 7.0) to prevent coagulation. The blood sample was centrifuged at 1500 rpm for 10 min at 4 °C in a clinical centrifuge, and the plasma and buffy coat were removed by aspiration. The erythrocyte pellet was washed thrice with phosphate buffered saline (PBS) and resuspended in PBS to give a 5% hematocrit.
Treatment of erythrocytes with TiO2 NPs
The reaction mixture containing 200 μL of erythrocyte suspension (ES). 0.1% Triton X-100, and 50, 100, 250 and 500 µg/mL of TiO2 nanoparticles were added to 96-well microplates. After that it was incubated for 3 hours at 37 °C under constant agitation. The release of hemoglobin was determined after centrifugation (6000 rpm for 15 min) by photometric analysis of the supernatant at 540 nm. Complete hemolysis was achieved using 0.1% Triton X-100 yielding the 100% (positive control) and PBS yielding the 0% (negative control) Hemolysis.
The % hemolysis was calculated as
Hemolysis (%) = (OD test sample – OD negative control) / (OD positive control – OD negative control) × 100. (1)
Results and Discussion
Structural XRD analysis
The XRD patterns of the as-prepared sample and annealed TiO2 nanoparticles synthesized by hydrothermal method are shown in Fig. 2(a), (b) and (c) respectively. The nanoparticles were calcinated between two different temperatures 450 and 500 oC / 4 h. Fig. 1 shows the XRD pattern of TiO2 nanoparticles with 2θ peaks lying at 25.6o (101), 37.8o (004), 48.1o (200), 53.9o (105), 56.0 o (211), 62.8 o (204), 75.1 o (220) & 83.2 o (215) reflections, respectively and confirmed the nanocrystalline nature of the synthesized particles. The XRD sample showed a dominant peak at 2θ = 25. 6o (101), which proved the crystallographic plane of anatase form of TiO2, which are closely in agreement with the standard (JCPDS NO: 89-4921). The particles size estimation was performed by the Scherrer’s formula:
D = kλ / βcosθ, (2)
where D is the mean diameter of the nanoparticles, K is the Scherrer’s constant with the value 0.94, λ is the wavelength of the X-ray radiation source, β is the angular full-width at half-maximum of the XRD peak, and θ is the Bragg angle. From the powder XRD spectrum the average crystallite size was found to be 13 nm.
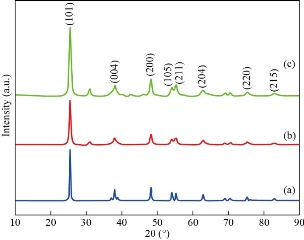
Fig. 1 XRD pattern of TiO2 nanoparticles for (a) as prepared sample, and (b) and (c) annealed samples.
Morphological FE-SEM and elemental EDX analysis
The surface morphology of the TiO2 nanostructure for as prepared and annealed samples was analyzed by FF-SEM and corresponding images are shown in Fig. 3. This image was observed within the magnification of 1 µm. Fig. 2(a)-(c) depicts the FE-SEM images of TiO2 nanoparticles which exhibited a spherical shape with little aggregations observed in the as-prepared and annealed sample. However, comparing the spherical structures of both samples it’s seen that particles sizes and morphology changes with the increasing in annealing temperature. The grain size of the particles 500nm, with increasing in sintering temperature. All the three samples similar morphologies were observed, it conform the presence of TiO2 nanoparticles. Energy dispersive X-ray spectra of synthesized TiO2 nanoparticles are represented in Fig. 2(d), which indicated the existence of oxygen and titanium. The energy dispersive X-ray analysis study (EDX) proves that the particles are crystalline in nature and indeed metallic TiO2 NPs.
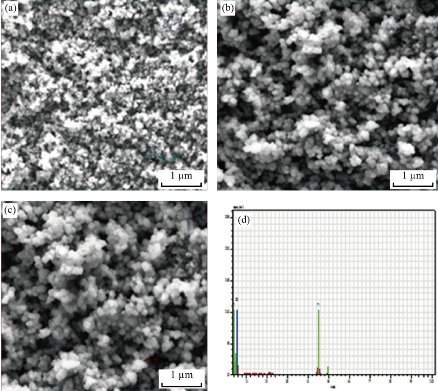
Fig. 2 FE-SEM images of TiO2 nanoparticles for (a) as prepared sample, (b) and (c) annealed samples, and (d) EDX spectrum showing the chemical composition.
Raman spectra analysis
Fig. 3 shows the Raman spectra of TiO2 nanoparticles for as prepared sample and annealed samples. The scattered light was filtered by a dielectric edge Rayleigh rejection filter at 100 to 1200 cm-1 line/mm diffraction grating. Fig. 4 shows the Raman spectra of pure TiO2 NPs which exhibited the anatase vibration modes centered at 149, 393, 507, and 631 cm-1. All of these bands are assigned to the anatase strong bands phase, and they can be attributed to the five Raman-active modes of anatase phase with the symmetries of Eg, B1g, A1g, and Eg, respectively. Both position of 149 cm-1 showed and confirmed the strongest anatase raman mode of TiO2 nanoparticles.
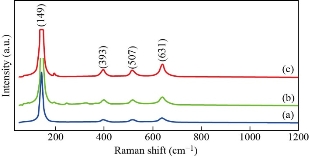
Fig. 3 Raman spectra of TiO2 nanoparticles for (a) as prepared sample, and (b) and (c) annealed samples.
FTIR functional group analysis
FTIR analysis was used to determine the functional groups of titanium dioxide (TiO2) nanoparticles. The FTIR spectra of as prepared and annealed TiO2 nanoparticles were analyzed in the range of 500 – 4000 cm-1 as shown in Fig. 4. Fig. 4 represents the FTIR absorption spectrum of the synthesized TiO2 nanoparticles which showed an intense peak at 3425 and 1631 cm−1 confirmed due to OH (hydroxyl) stretching and bending vibration mode. The bands at 1625 cm-1 are related to the presence of C-H bending and vibration frequency of O-H bond in water. Peaks below 800 cm-1 correspond to Ti-O (750 - 500 cm-1) and Ti-O-Ti (metal - oxygen) (530 cm-1) bending vibrations. The band located at 137 cm-1 corresponds to a vibration of Ti-ligand bond. It is known that the existence of hydrogen bonds between the hydroxyl results in the broad IR absorption band and the shift to higher wave number.
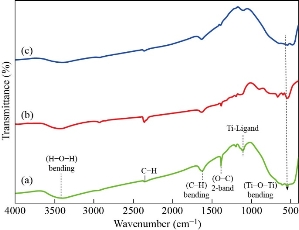
Fig. 4 FTIR spectra of TiO2 nanoparticles for (a) as prepared sample, (b) and (c) annealed samples.
Antimicrobial activity
The agar well diffusion method was performed against the B. subtilis, S. aureus, E. coli and P. aeruginosa, and the maximum zone of inhibition was observed in the TiO2 NPs against 4 different pathogens (Fig. 5). Table 1 shows the synthesized TiO2 NPs which displayed antibacterial activity of pathogenic strains of S. aureus (15 mm), B. subtilis (16 mm), E. coli (18 mm) and P. aeruginosa (17 mm) at 100 µg/mL, respectively. In the case of E. coli (gram-ve bacteria) shows high inhibition zone than the other B. subtilis (gram +ve), S. aureus (gram +ve) and P. aeruginosa (gram -ve) species in the zone inhibition range between 15 - 22 mm. The results were compared with the standard drug streptomycin, which suggests that the TiO2 NPs showed better antibacterial activity. The possible mechanisms are involved in the nano metal oxide (TiO2 NPs) carrying a positive charge and microorganisms carrying a negative charge. Hence, electromagnetic interactions between metal oxide and microorganisms lead to oxidation. Titanium nanoparticles are capable of dissolving the outer membranes of bacteria due to the presence of hydroxyl groups leading to the death of the organisms.
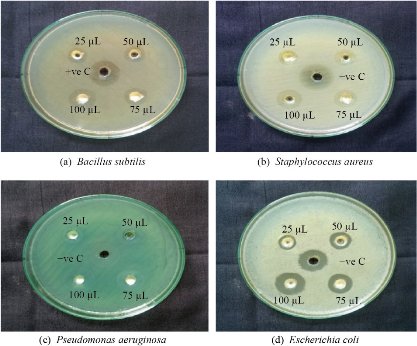
Fig. 5 The antimicrobial activity of TiO2 nanoparticles.
Table 1 Zone of inhibition of TiO2 NPs
|
Organisms |
Zone inhibition (mm in diameter) |
||||
|
25 µg/ mL |
50 µg/mL |
75 µg/mL |
100 µg/mL |
PC |
|
|
P. aeruginosa |
06 |
09 |
14 |
17 |
18 |
|
E. coli |
04 |
07 |
11 |
18 |
16 |
|
S. aureus |
05 |
08 |
12 |
15 |
22 |
|
B. subtilis |
05 |
09 |
13 |
16 |
17 |
In vitro hemolytic activity of TiO2 nanoparticles
The photograph (Fig. 6) shows the in-vitro biocompatibility of different concentrations of 50, 100, 250 and 500 μg/mL TiO2 nanoparticles showed the different percentage ranges such as 28.2, 32.5, 39.2 and 50.1% (Table 2). All nanomaterials enter into the blood get in contact with red blood cells (RBC). To assess the impact of TiO2 NPs on erythrocyte, hemolysis test was performed by spectrophotometric method. It was observed that the interaction of TiO2 nanoparticles with RBC revealed a hemolysis percentage of 500 μg/mL caused a significant range of higher level of hemolysis, exceeding 50.1%. The performance of hemolysis assay was tested by the negative control phosphate buffered saline (PBS) and positive control (Triton-X-100). The observed hemolysis properties of these synthesized TiO2 nanoparticles could be essentially attributed to their size, shape, surface chemistry and physiochemical properties. Hemolysis process involves the denaturation of cells through the physiochemical interaction between NPs and the cell surface. The results stated that TiO2 NPs showed lower hemolytic activity compared with positive control. The mechanism of the hemolytic activity of the NPs depends on the increasing permeability to complete lysis of the cell. The cell lysis caused free radical formation and cell death and resulted in harmless RBC cell count.
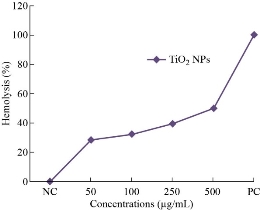
Fig. 6 The hemolytic activity of TiO2 nanoparticles.
Table 2 The hemolytic percentage of TiO2 nanoparticles
|
Sample concentrations (μg/mL) |
Hemolysis (%) |
|
50 |
28.2 |
|
100 |
32.5 |
|
250 |
39.2 |
|
500 |
50.1 |
Conclusions
The present study has demonstrated that the TiO2 NPs were successfully synthesized by hydrothermal method using titanium tetra isopropoxide and isoproponal.. They were calcinated between 450 ℃ and 500 ℃. The calcinated TiO2 nanopowders were characterized by powder XRD, FTIR, FE- SEM, EDX and Raman analysis. The powder XRD spectra revealed that the main phase of TiO2 nanoparticles were anatase phase and crystalline in nature. FTIR spectra of TiO2 nanoparticles showed an intense peak at 3425 and 1631 cm−1 confirmed due to OH (hydroxyl) stretching and bending vibration mode. Also, FTIR spectra showed the vibrational mode of TiO2 around 530 cm-1. The spherical shaped particles were studied by the FE-SEM analysis. Elemental analysis of the samples was investigated by EDX spectroscopy in order to confirm the presence of titanium and oxygen. The in-vitro antibacterial activity of TiO2 NPs was observed against both gram-positive and gram negative bacteria and the results showed that TiO2 NPs had potential inhibitory zone of inhibitions against E. coli and P. aeruginosa while there was less action against S. aureus and B. subtilis. Moreover, the TiO2 nanoparticles exhibited considerable antimicrobial activity against pathogenic bacteria, which is comparable with that of standard antibiotic. Nevertheless, a rare study has been conducted in this area that can provide a clear understanding of the toxic effect and mechanism of TiO2 NPs exposure to RBCs. The present study compared the adsorption, uptake and hemolytic potentials of different sizes of TiO2 NPs in the RBC cells. Based on these results, we conclude that the TiO2 nanoparticles may have potential biomedical applications due to its enhanced dispersibility, stability and surface coatings.
Acknowledgements
The authors are grateful to DST-FIST and UGC SAP, New Delhi, India for the instrumentation facilities.
Funding
Funding was provided by the Department of Science and Technology (DST) PURSE-Phase-II.
Conflict of Interests
All author(s) declare that they have no conflict of interest regarding the publication of this paper.
References
Copyright© Santhanakrishnan Mahalakshmi, Parthasarathy Vijaya. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.