Synthesis of Alpha-Gamma Aluminum Oxide Nanocomposite via Electrochemical Method for Antibacterial Activity
Ahmed Mahdi Rheima 1 *, Ahmed Abed Anber 2, Hussein Ismael Abdullah 3, Ahmad Hussein Ismail 3
1 Department of Chemistry, College of Science, University of Wasit, Kut, Iraq.
2 Department of Student Affairs and Registration, Al-Karkh University of Science, Baghdad, Iraq.
3 Department of Chemistry, College of Science, Mustansiriyah University, Baghdad, Iraq.
* Corresponding author. E-mail: arahema@uowasit.edu.iq
Received: Apr. 27, 2020; Accepted: Sep. 28, 2020; Published: Dec. 25, 2020
Citation: Ahmed Mahdi Rheima, Ahmed Abed Anber, Hussein Ismael Abdullah, and Ahmad Hussein Ismail, Synthesis of Alpha-Gamma Aluminum Oxide Nanocomposite via Electrochemical Method for Antibacterial Activity. Nano Biomed. Eng., 2021, 13(1): 1-5.
DOI: 10.5101/nbe.v13i1.p1-5.
Abstract
This work was devoted to synthesizing α-ɣ Al2O3 nanocomposite by the electrolysis method. A plate of aluminum was used as the anode, while the graphite rod was used as the counter electrode (cathode). The structure and morphology were investigated and characterized by X-ray diffraction (XRD) and transmission electron microscopy (TEM). The results indicated the average size of α-ɣ Al2O3 nanocomposite was smaller than 10 nm. The value of inhibition zone indicated the nanocomposite’s effect on different bacteria. The results demonstrated the newly synthesized nanocomposite as promising antimicrobial agents against bacteria.
Keywords: α-ɣ Al2O3 nanocomposite, TEM, Electrochemical method, Antibacterial activity
Introduction
Nanotechnology is developing as quickly with its application in science for the engineering of new materials at the nanoscale [1-7]. The possess of Nanoparticles are different chemical properties when compared to bulk kinds of similar chemical composition [8-11]. Nanotechnology has extensive interests, especially in the medical, industrial, and environmental fields [12-15]. Metal oxide nanoparticles have exhibited better stability, low poisonousness, high immovability, and selectivity associated with organic compounds [16-18]. Moreover, the particle size of such is responsible for the changes in their basic physical and chemical properties. These particles exhibit remarkable applications in catalysis, drug delivery, water treatment, sensor devices, semiconductor materials, and solid oxide fuels [19, 20]. Aluminum oxide nanoparticles have significant industrial applications [21], and they has many usages like abrasive material, as an absorbent in heterogeneous catalysis and metal-matrix composites as a biomaterial and reinforcements [22, 23]. A wide range of applications of Al2O3 was employed, such as sensors, electronics devices, antimicrobial, catalyst, and so on. [24,25]. Here it is many forms of crystallographic phases, on which γ and α forms were used for application because of its different features. The high surface area of γ-Al2O3 lead to it useful for catalyst related application [26,27], whereas the polycrystalline α-Al2O3 is widely used for glass-ceramic application [28,29]. It is outstanding from information in writing that for acquiring items of thick nanocrystalline Al2O3, both phases change from γ to α phase must be prevented or nanocrystalline α-Al2O3 powders must be utilized [30-32]. Aluminum oxide nanoparticles can be the preparation by several methods containing electrolysis, hydrothermal, sol-gel, spray pyrolysis, plasma sputtering, laser ablation, and pulse laser deposition [33–37]. The purpose of this work is to synthesis a novel nanocomposite of two phases of aluminum oxide (alpha and gamma) by electrolysis method, which is considered a new with a nanocomposite because whole methods were prepared one phase structure. There is not considered an important research effort on the antibacterial of α-ɣ Al2O3 nanocomposite. Thus the result can be considered high, and the work can be a good attempt to syntheses α-ɣ Al2O3 nanocomposite by electrochemical method and employ in such an important application.
Experimental
Chemicals and reagents
No further purification, both chemicals were of analytical reagent grades and used as obtained.
Synthesis of α-ɣ Al2O3 nanocomposite
α-ɣ Al2O3 nanocomposite have been synthesized by the electrolysis method, Fig (1), using 150 ml of 0.08 M KOH at 25 C° as the electrolyte. A rectangular aluminum plate (50 mm x 25 mm x 1 mm) used as the anode. Graphite rod (7 x 70 mm) used as the counter electrode cathode. Before mounting the substrates in the cell, they are cleaned sonically using organic cleaner and aqueous solvents (ethanol, acetone, chloroform, de-ionized water) Sequentially, and each cleaning step duration is 5 minutes. The applied d.c. The voltage between the electrodes is 15 V under a current density of 9.22 x10-3 mA\cm2 for 8 h, A brawn precipitate has obtained, and the product has been washed with de-ionized water and dried overnight to subsequent analysis.
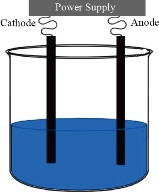
Fig. 1 The electrochemical cell.
Antibacterial assay
The α-ɣ Al2O3 nanocomposite synthesized using the electrolysis method. The nanocomposite was tested for antibacterial activity by agar disc diffusion method against bacillus anthrax, then incubated at 37 °C for 24 h. Finally, the inhibition zone of bacteria was measured at different levels.
Results and Discussion
Fig. 2 shows X-ray diffraction patterns of α-ɣ Al2O3 nanocomposite powders. It observes six crystalline peaks of ɣ-Al2O3, which have miller indices (220) (311) (222)(400)(511) and (440) [38], and five crystalline peaks of α-Al2O3, also have miller indices (012)(311)(024)(122) and (128) [39]. The crystalline size of α-ɣ Al2O3 nanocomposite has been calculated by using Scherer’s formula (D = 0.9λ/B cosθ)[40]. The mean crystallite size of nanoparticles was 9.1 nm, while the (222) reflection peak indicated the formation of ɣ-Al2O3. The morphology of α-ɣ Al2O3 nanocomposite powders has been characterized by transmission electron microscopy (TEM) in Fig. 3. The size of the nanocomposite is apparent with a size of less than 10 nm. The agglomeration of more nodular individual particles has existed in the structure of nanocomposite. Most of the particles have rough surface morphology. Fig. 5 shows the inhibition zone, which indicates the nanocomposite of α-ɣ Al2O3 shows the strong effect against gram-negative bacteria type bacillus anthrax. Hence, the results demonstrate that the α-ɣ Al2O3 nanocomposite promising antimicrobial agents against bacteria. The activity of nanoparticles against bacteria has been reported by several studies that were obtained the mechanism of the bactericidal effect of nanoparticles is not understood wholly. It may be the nanoparticle attack to surface membrane. Many changes took place in its membrane morphology and disturb its power function, such as permeability, produced an essential increase in its permeability, affecting suitable transport through the membrane of plasma, resulting in cell death. It can be that the penetration of nanoparticle inside bacteria causing damage via interaction with Sulphur and phosphorous having compounds such as DNA. It can vanish its replication ability, and cellular proteins convert an inactive after the treatment of nanoparticle. In this project, the strong activity of Al2O3 due to the small size of the nanoparticles provides good penetration of the nanoparticles inside the cell then caused many changes later cell death [1, 14, 16, 41].
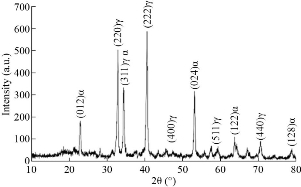
Fig. 2 XRD pattern of α-ɣ Al2O3 nanocomposite.
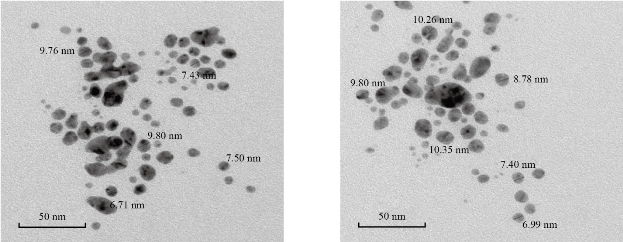
Fig. 3 TEM images of α-ɣ Al2O3 nanocomposite at different locations.
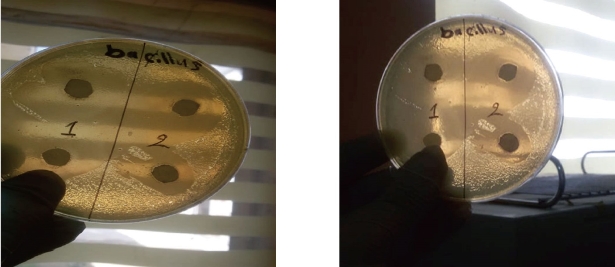
Fig. 4 The inhibition zone of bacterial growth on agar plates by using α-ɣ Al2O3 nanocomposite as antibacterial.
Conclusions
α-ɣ Al2O3 nanocomposite was synthesized by electrolysis method, which was a simple and efficient method for preparing α-ɣ Al2O3 nanocomposite. The prepared nanocomposite was characterized using X-ray diffraction analysis, field emission scanning electron microscopy, and transmission electron microscopy, which displayed that the as-prepared sample had the size of α-ɣ Al2O3 nanocomposite less than 10 nm. In addition, α-ɣ Al2O3 nanocomposite showed a strong activity against bacteria, and this activity was influenced by the size of nanoparticles due to which they could easily reach the nuclear content of bacteria. The nanoparticles presented large and impressive surface area; thus, the contact with bacteria was the greatest, which could be the reason behind the strong activity against bacteria, and that smaller particles made large inhibition zones.
Acknowledgments
We gratefully acknowledge the Faculty of Chemistry at Wasit University for supporting this work.
References
Copyright© Ahmed Mahdi Rheima, Ahmed Abed Anber, Hussein Ismael Abdullah, and Ahmad Hussein Ismail. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.