Histological, Biochemical and DNA Changes in the Liver of Male Albino Rats Treated with Silver Nanoparticles
Abed Hassan Baraaj *
Department of Biology, Collage of Sciences- University of Baghdad, Baghdad, Iraq.
* Corresponding author. E-mail: abed.hassan20@yahoo.com
Received: May 19, 2020; Accepted: Aug. 28, 2020; Published: Nov. 21, 2020
Citation: Abed Hassan. Baraaj, Histological, Biochemical and DNA Changes in the Liver of Male Albino Rats Treated with Silver Nanoparticles. Nano Biomed. Eng., 2021, 13(1): 20-26.
DOI: 10.5101/nbe.v13i1.p20-26.
Abstract
Sliver nanoparticles (Ag NPs) are produced industrially and commercially available in recent time. Silver and its nanoparticles are widely applied to consuming medical purposes. Silver nanoparticles are able to resemble cellular components and typical proteins, which helps the nanoparticles to cross natural barriers in human body. For this reason, the current study was designed to investigate the damage caused by silver nanoparticles, especially in liver which is one of the most targeted organs of nanoparticles as a result of their accumulation and damages caused in various aspects such as tissue, functional ability and DNA of liver cells. 30 albino male rats were used for this study and were divided into 3 main groups. The first group represented control and a normal saline solution was given, while the second group was given silver nanoparticles at a concentration of 2 mg/kg of body weight, and the third group was given silver nanoparticles at a concentration of 3 mg/kg of body weight. Each concentration of Ag NPs was dissolved in 3 mL of the normal saline and given directly to the experimental animal’s stomach by feeding tube with diameter of 0.6 cm and every day for 10 weeks. The results in the current study showed histopathological change in liver tissue such as large granulomatous lesion with an increase in macrophage numbers, as also shown by necrotic hepatocytes with dilation of sinusoids. The deformation of architecture in the parenchyma appeared in both concentrations. The biochemical parameters investigation in the liver clarified increased level of enzymes accordance with doses of Ag NPs. The damage was identified in DNA of hepatocytes by comet assay and the degree of damage was evident through increasing the numbers of tailed nuclei and in strand breaks of DNA. The reactive oxygen species (ROS) generation had a key role in the events of structural and functional disorders in the liver of experimental animals.
Keywords: Silver, Nanoparticles, Liver, Histopathological, Enzymes, Comet assay, DNA
Introduction
The technology that deals with structures and size of materials ranging in size from 1 - 100 nm nanoparticles is called nanotechnology; it has been shown that nanoparticles have a relatively higher toxicity when compared to other sizes. In addition, the different toxicity of nanoparticles is related to different sizes and shapes of nanoparticles [1]. Numerous studies and reports have been issued on the potential benefits of applying nanoparticles of silver (Ag NPs) in various areas of life, but there is a missing of Information related to health effects during home use of nanoparticles-based materials. Recent articles have confirmed that nanoparticles to be possible for penetrates the human body [2-4]. Nanoparticles have dimensions that make them resemble cellular components and typical proteins, which helps the nanoparticles to a cross natural barriers in human body, leading to harmful of tissues reactions [5]. Surface areas play a major role in determining the important properties of the toxic interaction of nanoparticles with in cells [6, 7]. Silver nanoparticles are one of the manufactured nanomaterials produced industrially and commercially available in recent time, silver and its nanoparticles are widely applied to consumer products and medical purposes [8]. The digestive system absorbs the nanoparticles of silver which are transferred to the liver by the hepatic portal vein and have harmful effect on the liver according to its work by conducting a preliminary examination of all the foods Absorbed by the digestive system. Before it turns into other system in the body, the nanoparticles of silver are bound with blood plasma proteins and then distributed to various organs of the body and enter the cells [3, 9]. Nanoparticles have various physical and chemical properties and interaction with cells by divergent way indirectly such as macro- pinocytosis, phagocytosis and endocytosis or directly via so-called adhesive reaction [10]. Important increase was observed in the level of liver enzymes in the blood of mice treated with silver nanoparticles due to the harmful physiological effect of these molecules when compared with control group as a result of severe irritation of the intracellular oxidation system due to the liver damage [9]. Silver nanoparticles or silver ions released from them interact with the biological system and cause oxidative stress of cells, leading to DNA damage and death of inflammatory cells [11].
Experimental
The preparation of Ag NPs
Silver nanoparticles (Ag NPs), nano powder particle size < 100 nm were manufactured by Sigma Aldrich Chemical Company (USA). nano powder of silver was weighted by sensitive balance to acquire 2 doses (2 and 3 mg) which agreed with the body weight of experimental rats [11]. Each weight of silver powder was dissolving in 3 mL of physiological solution (0.09 NaCl), and then given to experimental animals orally direct to the stomach via a feeding tube (0.6 cm in dimeter) for 8 weeks [12].
Experimental animals
The experiment was carried out in the current study on 30 male sexually mature Sprague-Dawley albino rats (Rattus norvegicus). The weight of the experimental male rats ranged from 200 - 220 gm and their age at 16 - 17 weeks. The animals were obtained from the pharmaceutical control center in Baghdad. The animals were kept under appropriate laboratory condition and adapted to laboratory conditions for a period of 15 days from the date of their establishment. 30 male rats were used, divided into 3 main groups randomly, by 10 rats per group with dosed as follows:
Group 1: Control, rats were given orally with physiological solution (0.09 NaCl) for 10 weeks;
Group 2: Treated with Ag NPs suspension, rats were given orally with 2 mg/kg b.w for 10 weeks; and
Group 3: Treated with Ag NPs suspension, rats were given orally with 3 mg/kg b.w. for 10 weeks.
Dealing with animals
The experimental male rats were killed under anesthesia after completing the experiments on them. The collected blood samples were centrifuged at 3000 rpm for 20 min. The blood serum was kept at -20 ℃, with liver extract of each male rat.
Samples prearations
To achieve the purpose of this study, different preparations of samples were required for the histological, biochemical and DNA aspects to assess the extent of the harmful effect of silver nanoparticles on the hepatocytes of experimental male rats as follows.
Histological preparation
It was performed on liver samples in size 0.5 cm for each sample; slides were prepared and stained for histological diagnosis according to [13].
Biochemical preparation
The estimation of liver enzymes activity was conducted, including:
Estimation of serum activity of alanine aminotransferase (ALT) adopting the procedure with ALT kit from Agappe Company (India);
Estimation of serum activity of aspartate aminotransferase (AST) adopting the procedure with AST kit from Agappe Company (India); and
Estimation of serum activity of alkaline phosphatase (ALP) adopting the procedure with ALP kit from Agappe Company (India).
Comet assay
To determine the amount of DNA damage in the liver cells of experimental male rats, the comet assay kit (Trevigen) was used and performed with a test according to Boeck et al. [14].
Statistical analysis
Statistical analysis of the results obtained from the experiments of this study was carried out to compare the study groups, taking into consideration the effectiveness of liver enzymes by the program of the statistical analysis system 2016. Testing was applied to analysis of variance (ANOVA). The results were expressed with mean standard deviation (Mean + S.D., statistically analyzed at a probability level less than 5% (p < 0.05) [15].
Results and Discussion
Animals dosing ended with silver nanoparticles for 10 weeks and the following results appeared in the histopathological study. For animals dosed with Ag NPs (2 mg/kg b.w.), the sections of liver disclosed neutrophils and mononuclear cells aggregated near the lumen of blood vessels with macrophages in liver parenchyma (Fig. 2). In addition, monocytes and neutrophils aggregated in portal area with proliferation of Kupffer cells and necrotic hepatocytes (Fig. 3), which was shown when compared with histological section of the control group liver (Fig. 1). For animals dosed with Ag NPs (3 mg/kg b.w.), the sections of liver exhibited granulomatous lesion in the parenchyma with necrotic hepatocytes (Fig. 4). It also showed congested blood vessels with fibrosis in portal area and dense dark brown plugs of bile in the canaliculi, as well as ballooning degeneration in the hepatocytes (Fig. 5). This study clarified that both doses caused deformed liver parenchyma architecture compared with histological sections of the control group liver (Fig. 1).
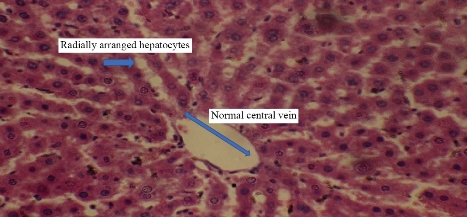
Fig. 1 Cross section in the liver of control male rats showed radially arranged hepatocytes with normal central vein (H&E stain 40 ×).
The immune system keeps the human body from harmful effects such as environmental pollutants, microbial infected and manufactured ones. The above results were consistent with previous study, pointing out that due to interacting with nanoparticles which led to immunotoxicity, phagocytosis played an important role in addressing the nanoparticles recognized treated and eliminated [16]. The present study showed the cluster of different kinds of immunity cells in liver, which was in agreement with numerous previous research, pointing out that when increasing the percentages of mononuclear cells, macrophages and neutrophils, especially in the liver when exposed to the accumulation of nanoparticles [17, 18]], these cells accumulated around the blood vessels and in the parenchyma of liver exposed to nanoparticles [16]. The appearance of bile plug in this study was consistent with the result found by Shrivastava et al. [19].
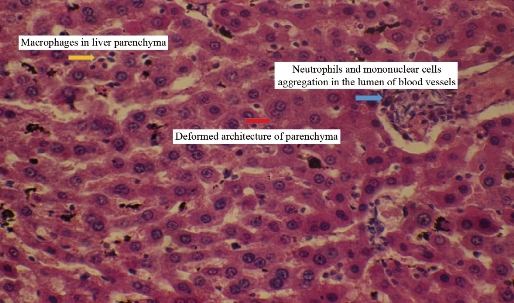
Fig. 2 Cross section in the liver of male rats treated with 2 mg/km Ag NPs showing neutrophils and mononuclear cells aggregation in the lumen of blood vessels with macrophages in liver parenchyma with deformed architecture of parenchyma (H&E stain 40 ×).
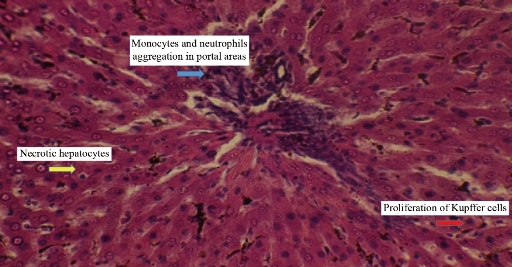
Fig. 3 Cross section in the liver of male rats treated with 2 mg/kg Ag NPs exhibiting monocytes and neutrophils aggregation in portal area with proliferation of Kupffer cells and necrotic hepatocytes (H&E stain 40 ×).
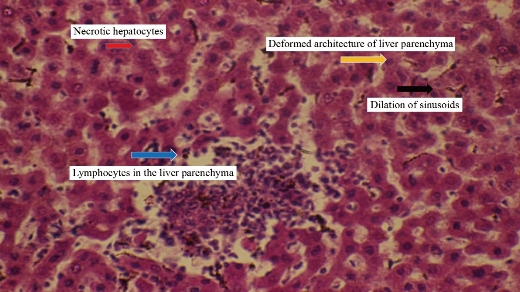
Fig. 4 Cross section in the liver of male rats treated with 3 mg/kg Ag NPs showing large granulomatous lesion consisting from aggregation of active macrophages and lymphocytes in the liver parenchyma with necrotic hepatocytes, also exhibiting deformed architecture of liver parenchyma with dilation of sinusoids (H&E stain 40 ×).
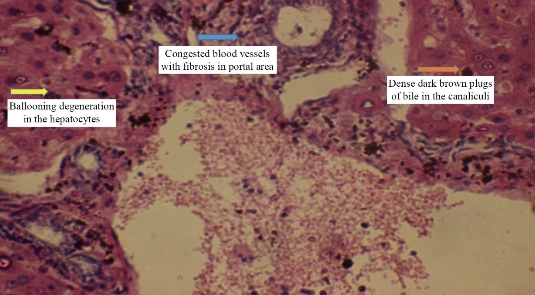
Fig. 5 Cross section in the liver of male rats treated with 3 mg/kg Ag NPs exhibiting congested blood vessels with fibrosis in portal area and dense dark brown plugs of bile in the canaliculi with ballooning degeneration in the hepatocytes (H&E stain 40 ×).
Biochemical study
The result in the current study showed an increased level of liver enzymes (ALT, AST, ALP) when experimental animals are exposed to different concentrations of silver nanoparticles as shown in Table 1, which corresponded to pathological changes in liver tissue. The liver is one of the most important organs targeted by the silver nanoparticles, accumulating in it and causing histological and functional damage to the liver [20]. The above results were in agreement with those reported by Monfared et al. [21]. For this reason, the increased level of these enzymes was an indication of the occurrence damage to the liver [22, 23]. According to the findings by Park et al. and Lee et al. [24, 25], the recognized hepatotoxicity by alteration of liver function through biochemical parameters changed in blood serum of treated groups.
Table 1 Effect of Ag NPs on enzymes level of livers
|
Groups treatments |
Mean ± SD |
||
|
ALT (IU/L) |
AST (IU/L) |
ALP (IU/L) |
|
|
Control |
31.62 ± 3.04 |
44.34 ± 5.71 |
200.3 ± 10.31 |
|
AgNPs 2mg /kg |
41.37 ± 4.21 |
60.38 ± 5.53 |
238 ± 10.9 |
|
AgNPs 3mg /kg |
43.21 ± 5.91 |
62.85 ± 7.71 |
264.32 ± 12.95 |
|
LSD |
2.19 |
3.20 |
18.92 |
|
Significant difference (p ≤ 0.05) between groups |
|||
Comet assay
Fig. 7 shows the DNA damage in liver cells of male albino rats that were treated with silver nanoparticles in both doses for 10 weeks, and the degree of damage was evident by increasing the numbers of tailed nuclei and in strand breaks of DNA when compared with the control group (Fig. 6). The current study suggested that dealing with silver nanoparticles stimulated gene mutation, necrosis, strand breaks of DNA and reactive oxygen species generation (ROS). According to previous studies, the excessive generation of reactive oxygen species caused necrosis and apoptosis [26, 27]. An increased level of ROS was connected with massive damage of DNA with necrotic increase [28, 29]. In addition, previous investigation pointed out that silver nanoparticles stimulated cytotoxicity and genotoxicity in cells [30], decreasing viability in different cells and generation of ROS causing necrosis and apoptosis by the pathway of mitochondria [31, 32] which caused lipid peroxidation on biological membranes with harmful to DNA and structure proteins [33, 34].
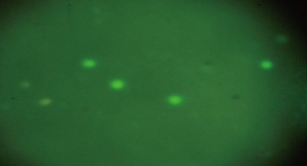
Fig. 6 Scoring categories of comet assay showing no damage in hepatocytes of the control group of male albino rats.
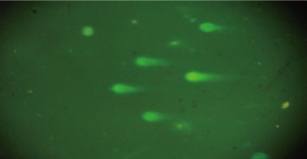
Fig. 7 Scoring categories of comet assay showing high damage increased the numbers of tailed nuclei and in strand breaks of DNA in hepatocytes of groups treated with Ag NPs of male albino rats.
Conclusions
Silver nanoparticles caused damage to liver induced various histopathological changes with deformed architecture of hepatocytes, and caused alteration in the biochemical parameter of liver function such as AST, ALT and ALP. Silver nanoparticles induced DNA damage of liver through cytotoxicity effect of Ag NPs interaction with DNA. The size of Ag NPs played an important role to harm the liver because it was easily absorbed by small intestine and transferred to the liver, and then accumulated there, causing various damages in the liver of the treated groups of male albino rats. Silver nanoparticles showed the ability to induce generation of reactive oxygen species, necrosis apoptosis, cytotoxicity and genotoxicity in cells, with immune disorders also occurring.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Abed Hassan. Baraaj. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.