Research Article
Comparative Killing Activity of Different Nanoparticles and Nano-composites Based on Dermanyssus gallinae
Sameh Ismail 1 *, Gehad Mohamed 1, 2, Aziza Amer 3, Mohamed Amer 4
1 Egypt Nanotechnology Center, Cairo University, El-Sheikh Zayed, 6th October, Giza, Egypt.
2 Chemistry Department, Faculty of Science, Cairo University, 12613, Giza, Egypt.
3 Department of Pharmacology, Faculty Veterinary Medicine, Cairo University, 12211 Giza, Egypt.
4 Department of Poultry Diseases, Faculty Veterinary Medicine, Cairo University, 12211 Giza, Egypt.
* Corresponding authors. E-mail: drsameheltayer@yahoo.com
Received: Jan. 10, 2020; Accepted: Oct. 10, 2020; Published: Nov. 27, 2020
Citation: Sameh Ismail, Gehad Mohamed, Aziza Amer, and Mohamed Amer, Comparative Killing Activity of Different Nanoparticles and Nano-composites Based on Dermanyssus gallinae. Nano Biomed. Eng., 2020, 12(4): 338-350.
DOI: 10.5101/nbe.v12i4.p338-350.
Abstract
Silver (Ag NPs) and magnetite nanoparticles (MANPs) were synthesized and characterized using X-ray powder diffraction (XRD), X-ray fluorescence (XRF), scanning electron microscope (SEM) and transmission electron microscope (TEM), atomic force microscope (AFM) and Raman spectroscopy. XRD, XRF and Raman spectroscopy results of MANPs and Ag NPs confirmed their synthesis without any undesired impurities from synthesis method. TEM, SEM and AFM images of Ag NPs illustrated homogenous spherical particles with the size of about 5 nm, while MANPs had spherical to semi-cubic with the size of 20 nm. Both silver-essential oils and magnetite–essential oils nanocomposites were synthesized by sonochemical method with the ratio of 1:1. In-vitro contact effect of commercial plant essential oil mixture with synthetic silver and magnetite nanoparticles was studied. Comparative killing study was carried out by direct contact spray of groups of saline, silver nanoparticles, magnetite nanoparticles, essential oils, silver-essential oil nanocomposite, and magnetite-essential oil nanocomposite on poultry red mite Dermanyssus gallinae (D. gallinae). Activity and changes occurred were examined under stereomicroscope for 3 h. Interestingly, highest killing activity was obtained with silver-essential oil nanocomposite and silver nanoparticles in comparison with magnetite nanoparticles, essential oil and magnetite-essential oil nanocomposite. As a result, silver-essential oil nanocomposite and silver nanoparticles could be beneficial and involved in D. gallinae (red mite) control strategies in poultry industry.
Keywords: Red mites, Nanosilver, Nanomagnetite, In-vitro study, Contact, Plant oil mixture
Introduction
Dermanyssus gallinae (D. gallinae) infestation still represents a major threat to the egg production industry, posing serious animal health and welfare problem in poultry industry all over the world; the economic the egg production industry, adversely affecting productivity, impacting public health and their role as a disease vector are well known [1]. The veterinary and human medical impact needs adequate therapeutic measures to control this parasite [2-4]. This blood feeding mite causes production losses due to irritation and anemia, but may even cause death of its host and is also involved in transmission of many pathogenic agents responsible for severe outbreaks in both animals and humans [5,6]. Repeated use of various synthetic contact acaricides such as permethrin, carbaryl, diazinon, dichlorvos are the most commonly used for control D. gallinae infestation leads to drug chemical pollution. The development of resistance [1] and further, the residues in eggs and meat are a highly important problem for human health [7,8]. Route avoidance of these drawbacks could be the use of vegetable organic pharmaceuticals [9]. There is constant need for alternative control measures to maintain a good animal health in aviary systems [10]. In the last thousand years ago, plant extracts was used as acaricide/ insecticide in Asia. Plant essential oils may be an alternative source of products used in the treatment of mite infestations, because they are rich sources of bioactive chemicals [11]. Kim et al. [11] studied the activity of 56 plant essential oils against poultry collected adult D. gallinae using direct contact and fumigation methods and concluded that the effect of these essential oils was largely due to action in the vapour phase. The acaricidal effect of eleven essential oils against poultry red mite D. gallinae was tested in vitro using the direct contact method. The results revealed that oils of sweet basil, coriander peppermint and summer savory were the most effective [12]. Neem oil, pure garlic juice, eucalyptus essential oils, and wood vinegar reduced, killed and control red mite [13-16]. Garlic oil has medicinal use and pesticidal effects [17]. Nanotechnology has become one of the most promising new generations for pest control in the recent years [18], could provide cost-effective solution to some of the most challenging environmental cleanup problems [19], help to produce new pesticides, insecticides and insect repellants [20]. Silver nanoparticles were approved by many agriculture researchers as insecticides which highly affect both adults and larvae [21] and also for insect control [22]. One proposed mechanism by which Ag NPs produced toxicity is by enhancing intracellular levels of reactive oxygen species (ROS). ROS, when formed, produce subsequent cellular damage such as disrupting membrane integrity and damaging proteins and DNA [23]. Consumption of Ag NPs was resulted in cuticular demelanization in Drosophila [24]. Adult Tetranychusurticaespider mite mortality using different concentrations of silver nanoparticles by leaf spray showed that at 100 ppm concentration, more than 50% of mites were died while using leaf dipping methods, spraying with 3000 ppm concentration showed a mortality effect more than 90% [25]. Magnetite nanoparticles have antibacterial properties for both Gram positive and negative strains [26]. The present study was planned to synthesis silver nanoparticles, magnetite nanoparticles, silver–essential oil nanocomposite and magnetite-essential oil nanocomposite and evaluating their killing activity with respect to saline and essential oils by direct contact or spraying them on mites and observing the changes tooks place under stereomicroscope.
Experimental
All used chemicals are laboratory grade including ferric chloride hexahydrate (FeCl3.6H2O), ferrous chloride (FeCl2), tri-sodium citrate (TSC), polyethylene glycol (PEG 600) and AgNO3 manufactured by Sigma Company, India. Ammonia solution (34 %) was supplied from Al-Nasr Company, Egypt.
Red mite collection
Three hundred and sixty red mites (D. gallinae) of different stages were collected from naturally infested 5 poultry lying flocks. Mites were collected with the aid of a brush in plastic jars and were used for tests within 2 days of collection. Until testing, the mites were kept at 24 °C under a photoperiod of 16:8 h (light/dark). The collected mites were used in 3 replicates for testing of efficacy of mixture of vegetable oil extracts (Allisal®) alone or with either silver or magnetite nanoparticles solutions.
Essential oils
Allisal® commercial: a liquid supplement product of Envisal GmbH, Eulenbusch 10a, 21391 Repented, Germany. Allisal was an aqua suspension of natural plant oils composed of 2.5% garlic oil, 4.2% rosehip oil, 4.8% rapeseed oil and 14% polysorbate. It was used at a rate of 0.4% dilution in deionized water for testing its in vitro effect on red mites.
Synthesis
Synthesis of Ag NPs
Synthesis of Ag NPs was done by co-precipitation method using TSC as reducing agent and capping agent in the same time [29]. AgNO3 solution (0.01M) was dissolved in 100 mL deionized water, heated to boiling then was added 0.1 M TSC drop by drop with constant stirring and heated until the color of mixture become pale yellow. The solution was cooled to room temperature under dark condition to avoid lights.
Synthesis of MANPs
MANPs were synthesized also by co-precipitation method using ammonia as reducing agent and PEG 600 as capping agent [30]. FeCl3.6H2O solution (0.2 M) was added to 0.1M FeCl2 solution and the mixture was heated with constant stirring at 60 ºC for 15 min until orange solution was formed without any powder precipitate. Then, ammonia was added drop by drop until all solution become black. Finally, the pH was adjusted to 12 by adding more ammonia solution. Wash using deionized water and external magnets several times then adding PEG to the final powder and the mixture was subjected to sonication for 1h in condition of 0.2 cycle and 10% amplitude.
Synthesis of silver-essential oil nanocomposite
3 mL of 75 ppm silver nanoparticles was added to 3 mL essential oil then the mixture was subjected to sonication under condition of 0.5 cycle and 50% amplitude for 20 minutes until pale yellow color was obtained.
Synthesis of magnetite-essential oil nanocomposite
3 mL of 100 ppm magnetite nanoparticles was added to 3 mL essential oil then the mixture was subjected to sonication under condition of 0.1 cycle and 10% amplitude for 30 minutes until brown color was obtained.
Characterization of nanoparticles
The aim of characterization of MANPs and Ag NPs was to determine physiochemical properties which influence their bioactivity [31]. Characterization was classified into three sectors namely composition, microscopic and physical sectors. Composition sector was carried out by XRD (D8 Discovery–Bruker Company) at condition of 40 KV and 40 AM (1600W) at speed scan 0.01 and 2theta(θ) range from 10◦ to 80◦, XRF (X-Met 8000 Oxford Instruments Company.) and Raman spectroscopy (Lab. RAM-HR Evolution Horiba Co.) with acquisition time 5 sec (for Ag NPs) and 7 sec (for MANPs), accumulations 5 (for Ag NPs) and 1 (for MANPs) without spike filter and delay time and objective was X50 (for Ag NPs) and X100 (for MANPs) with grating 1800 (450-850 nm) and ND filter 50% (for Ag NPs) and 5% (for MANPs). The laser type was green of 532 nm. Microscopic sector was carried out by transmission electron microscope (TEM) model EM-2100 High-Resolution at magnification 25X and voltage 200 kV. Scanning electron microscope (SEM) was carried out by Jol 2000, Japan and atomic force microscope (AFM) was done using AFM 5600LS Agilent Technology Company. Physical sector was carried out to determine the surface area, pore volume pore size and, average particles radius by surface area and pore size analyzer model Nova Touch LX2 manufactured by Quantachrome Company. Killing activity observed under stereo microscope (model M165 C - Leica Biosystems Company). DLS and zeta potential were carried out by instrument manufacture by Malvern instruments Ltd. Model of Nano Sight NS500 to determine the size and zeta potential of nanoparticles and nanocomposites. Optical and magnetic properties for nanoparticles and nanocomposites were measured by spectrophotometer model of Shimadzu d50 and vibrating sample magnetometer (VSM) instrument model 8600 series manufactured by Lake Shore Cryotronics, Inc. Killing activity was observed under stereomicroscope (model M165 C - Leica Biosystems company).
In-vitro study
Grouping and treatment using direct contact with mixture of plant oils and/or MANPs and Ag NPs solutions was described. Sex groups, 20 mites each, were transferred to separate glass petri dishes and exposed to the following treatments: Group 1 kept as non-treated control. Group 2 was sprayed with 4% essential oil. Groups 3 and 4 were sprayed with MANPs (100 ppm/mL) and Ag NPs (75 ppm/mL), respectively. Group 5 was sprayed with magnetite-essential oil nanocomposite (1 mL of 75 ppm silver and 1 mL essential oil). Finally, for group 6, magnetite-essential oil nanocomposite (1 mL of 100 ppm magnetite and 1 mL essential oil) was used. All treated mite groups were observed under stereomicroscope at 1, 2 and 3 h after treatment where non-moving mites considered dead. The number of dead and alive mites was counted in all the plates. The efficacy (E) of each tested material at each observation time was calculated as follows:
E% = ((A – B)/A) × 100.
where A is the number of mites before spray and B is the number of mites after spray.
The time for parasite death was recorded and photos taken at different times from start of treatment until complete death of the parasites for comparative evaluation of their acaricidal effect.
Results and Discussion
XRD results of MANPs and Ag NPs showed the formation of MANPs and Ag NPs without any shifting of its 2θ peaks position or presence of peaks of another chemicals used through synthesis process which indicated the purity of MANPs and Ag NPs (Fig. 1). The XRD results gave information about crystallinity and crystal form of the materials. The XRD curve of Ag NPs showed best amorphous state without any additional peaks indicated the homogeny of chemical composition (there are no other peaks of chemicals that used in synthesis process). XRD curve of MANPs (Fig. 1) illustrated the best fit 2θ peaks position with cubic crystal form for magnetite nanoparticles according to ICCD data base (COD1011032 Fe3O4 Magnetite). Elemental analysis using Eva software from Bruker Company using XRD chart showed the composition of MANPs as 27.6% oxygen and 72.4% iron. XRD measure for Silver-essential oil nanocomposite and magnetite-essential oil nanocomposite was carried out to identify if there is any change in chemical composition of silver and magnetite nanoparticles by nanocomposite synthesis. XRD pattern of both silver – essential oil and magnetite – essential oil nanocomposites as shown in Fig. 2 and 3 illustrated unchanged in crystalline or chemical composition of both silver and magnetite nanoparticles by nanocomposite synthesis with essential oils. XRF charts illustrated the purity of MANPs and Ag NPs (Fig. 4 and 5) with percentage of Ag and Fe more than 97%. The purity allowed evaluating their bioactivity without any influence from any factors. Raman spectrum of Ag NPs (Fig 6) showed very sharp peak at 56.02 cm-1 and weak one at 146.41 cm-1. Raman spectrum of MANPs showed very sharp peak at 670 cm-1. TEM image showed the mono dispersion of MANPs particles with size about 20 nm and shape of spherical to semi-cubic (Fig. 7). TEM image of Ag NPs showed the best spherical shape with homogeneity size about 5 nm (Fig. 8). Fig. 9 pointed out the 2D and 3D AFM images regarding the shape, size, homogeneity of particles distribution and roughness profile of MANPs and Ag NPs, respectively. Thin films of MANPs and Ag NPs were prepared in order to give complete ability study under AFM was done by sonication the MANPs and Ag NPs suspension in deionized water for 1h at condition of 0.1 cycle and 15% amplitude then using Spain coater which make thin films on glass slid substrate. All images were of the size 500 X 500 nm using contact mode with speed of 0.1 in/sec and servo condition of I gain 0.5, P gain 2.5 and set point zero. 2D and 3D AFM images of Ag NPs and MANPs illustrated the spherical shape with well sorting without any agglomeration and homogenous in size distribution (2-7 nm for Ag NPs and 15-22 nm for MANPs) and shape. 2D and 3D AFM images of MANPs- essential oil and Ag NPs – essential oil nanocomposites illustrated the effect of oils in aggregation and size of silver and magnetite nanoparticles. However, 2D and 3D AFM images (Fig. 10 and 11) illustrated the homogenous of size distribution become bad sorting due to coating of oils to silver and magnetite nanoparticles formed different sizes of them but still aggregate in nano-size. DLS results as shown in Fig. 12 illustrated the size of nanoparticles effect by nanocomposite formation due to coating of essential oils to nanoparticles lead to agglomeration or concentration of nanoparticles. However, nanoparticles size was 5.3 nm and 21.7 nm for silver and magnetite nanoparticles, respectively. Nanocomposite size was 20.5 nm and 40 nm for Ag NPs – oil nanocomposite and magnetite -oil nanocomposite, respectively. The results of DLS were in good agreement with AFM results. Remarkable results illustrated by measure zeta potential of nanoparticles and nanocomposites where the zeta potential of Ag NPs and MANPs desperation in distilled water was -30 and -17 mV where Ag NPs-oils nanocomposite and MANPs-oils nanocomposite (nanoparticles desperation on oils) was -55 and -28 mV. Increased value of zeta potential of nanocomposite formed allowed them to increased its stability in oils and more desperation in oils than water and also increase surface charge. However, increased zeta potential value enhancement the killing activity of nanomaterials. Vibrating sample magnetometer (VSM) was carried out to illustrate the magnetic properties of MANPs and MANPs – essential oil nanocomposite and explain if there are changes in magnetic properties of nanoparticles after nanocomposite synthesis. VSM was measured at room temperature as shown in Fig. 13 and 14. Coercivity (Hci), magnetization (Ms) and retentivity (Mr) were 58.543 G, 39.370 emu/g and 2.1261 emu/g for MANPs, respectively, and 51.241 G, 32.11 emu/g and 2.01 emu/g for MANPs – oil nanocomposite, respectively. Both of them have S-shape which indicated the super para magnetic nature of them. However, nanocomposite synthesis does not effect on magnetic properties which explain why MANPs and MANPs – oil nanocomposites has almost the same killing activity. Mites were directly subjected to different treatments and examined under stereomicroscope until complete stop movements and their features were recorded at 1 and 2 h after treatments (Fig. 15). The non-treated mites showed active movement under stereomicroscope with pale light brown color at 1 and 2 h after treatment. The sprayed mites with 0.4% solution of essential oil were completely stopped movements which considered dead within 1 and 2 h after treatment with completely stretched legs and white bead like spots of oils accumulation on legs and bodies. Mites treated with MANPs (100 ppm) stopped movements after 2 h treatment and showed bent legs under their bodies with dark brown color on their bodies. Mites treated with Ag NPs (75 ppm) were directly stopped movement after treatments for 1 and 2 h with detached legs and white coloration and pale hit film surround the mites. Mites treated with magnetite – essential oil nanocomposite showed stop movement, detached legs and abnormal keratin surface of dark brown color after 2 h treatment. While, mites treated with magnetite – essential oils nanocomposite suddenly stop movements after spraying and showed complete distraction and detachment of legs and distracted body keratin with white coloration. Results of essential oil, Ag NPs, MANPs, magnetite – essential oil nanocomposite and silver – essential oil nanocomposite sprayed on D. gallinaeat with different doses were listed in Table (1). For the individual 0.4% essential oil, Ag NPs and silver – essential oil nanocomposite after 1, 2 and 3 h of contact showed strong acaricide effect of 100% red mites’ death at 1, 2 and 3 h. While, magnetite – essential oil nanocomposite showed 70.33±0.47% at 1 hour and 100% at 2 and 3 h contact. MANPs (75 ppm) alone showed slower acaricide effect started with 40.66 ±0.49% at 1 h followed by 100% at 2 and 3 h contact. XRD, XRF and Raman spectra results of MANPs and Ag NPs confirmed their synthesis without any contamination or undesired chemicals during synthesis methods, so the silver and magnetite nanoparticles used individual or in synthesis of nanocomposite with essential oil were pure. TEM, SEM and AFM images showed the mono desperation of MANPs particles size about 20 nm with spherical to semi-cubic shape and Ag NPs showed spherical shape with homogeneity size about 5 nm. The sharp peak at 56.02 cm-1 and weak one at 146.41 cm-1 found in the Raman spectrum of Ag NPs were characteristic to Ag lattice vibrational mode while other week peaks for oxygen bond due to high intensity of laser (ND filter 50%) may be led to formation of silver oxide. Raman spectrum of MANPs was carried out using acquisition time 7 sec, accumulation 1 without spike filter and delay time. Objective was X100 with grating 1800 (450-850 nm), ND filter 5% and the laser type was green of 532 nm. It was obvious from the MANPs Raman chart that the peak at 670 cm-1 was characteristic to magnetite lattice vibrational mode. TEM image showed the mono desperation of MANPs particle size about 20 nm and TEM image of Ag NPs showed spherical shape with homogeneity size about 5 nm. The 2D and 3D images of Ag NPs illustrated the spherical shape, while MANPs particles were of spherical to semi-cubic in shape. D. gallinae or the poultry red mite is a cosmopolitan hematophagous mite, parasitic on birds. D. gallinae life cycle possessed five stages (egg, larva, protonymph, deutonymph and adult). Infestation can triple its numbers in only 10 days where nymphs need a blood meal for metamorphosis and adult females need blood meals for egg maturation. The mites are small and grey in color, but may appear if they have filled with blood after feeding on the bird. The susceptibility of recent field collected D. gallinae from naturally infested commercial chicken houses to essential oil mixture and/or prepared nanoparticles as acaricide was evaluated. Normal color is grey in color and appears red after feeding on the bird. The outer part of the mite exoskeleton, known as the epicuticle, consists of a layer of wax, which further limits water loss, and a cement layer, which protects the cuticle from external abrasion [32]. The susceptibility of collected D. gallinae to essential oil, MANPs, Ag NPs, magnetite – essential oil nanocomposite and silver – essential oil nanocomposite as acaricide was carried out by direct spray to fulfill contact toxicity [33]. The non-treated mites showed active movement under stereomicroscope with pale grey color at 1 and 2 h. The mites are grey in color, but protonymph, deutonymph and adult appeared red after feeding on the bird. The mites sprayed with 0.4% oil solution were completely stopped movements and considered dead after 1 and 2 h contact with completely stretched legs with white bead like spots of oils accumulation on legs and bodies. The used essential oil mixture (Allisal®) contains garlic oil, rosehip oil and rapeseed oil. Researchers [34-36] stated that plant-derived essential oils are shown to have a lethal characteristic, where garlic and thyme oils were the most effective. In the other side, the synergistic action of essential oil for cuticular penetration was reported [37, 38]. Mites treated with MANPs of 75ppm concentration as spray were stopped movements after 2 h and showed bent legs under their bodies with dark brown color. Silver nanoparticles have a significant impact on insect [39]. Also, Ag NPs were resulted in cuticular depenalization in Drosophila. The dark brown spot can be attributed to accumulation of Ag NPs as they were apo plastically transported in the cell wall and found aggregated at plasmodesmata [40] or can die due to Ag NPs induced oxidative stress as indicated by histochemical staining of superoxide radical and hydrogen peroxide that was manifested in terms of DNA degradation and cell death [41]. Mites treated with 75 ppm Ag NPs directly stopped movement (5 sec) and lasted at 1 and 2 h with detached legs and white coloration and pale hit film surround the mites, while those treated with magnetite – essential oils nanocomposite showed stop movement and detached legs and abnormal keratin surface of dark brown color at 2 h. While, mites treated with silver – essential oils nanocomposite suddenly (2 sec) stopped movements after spraying and showed complete distraction and detachment of legs and distracted body keratin with white coloration. This result proved the synergistic acaricidal action of essential oil and nanoparticles. Regarding the acaricidal effect measured by percent of D. gallinae deaths in relation to time of exposure. The 0.4% essential oils solution (garlic oil, rosehip oil and rape seed) showed strong acaricide effect with 100% deaths at 1, 2 and 3 h. This result was previously reported by many researchers. Carvacrol (essential oil of Origanum vulgare), garlic extract, cinnamon, eucalyptus and mint extract reduction in mite control with 92%, 96%, 66.97, 80.85 and 90.19%; respectively. Essential oils were tested for the movable D. gallinae acaricidal activity regardless of the stage of their development. The results were 80% rosemary oil (>90% mortality), 50% lavender (75% mortality), 100% thyme (80% mortality) and 80% clove oil (73% mortality) as measured after 48h [42]. Garlic extract was 96% effective after two successive sprays. Acaricidal activity of plant bioactive components was assessed against D. gallinae by contact toxicity carvacrol and thymol were found to be toxic to D. gallinae with LD50 values of 1 and 3.15 μg/cm, respectively [42]. Combination of carvacrol-thymol in 4:1 ratio at 2% concentration displayed good residual toxicity and was effective against D. gallinae till 14 days post spraying and a synergistic effect can be suggested for the control of D. gallinae [43]. The main chemical constituent of intact garlic is the amino acid allicin, an alkyl derivative of cysteine alkyl sulfoxide, which may vary from 0.2 to 2.0% fresh weight [44-47]. Garlic contains at least 100 sulfur-containing compounds basic to medicinal uses. Allicin represents 70-80 % of the total thiosulfates [47-50]. Garlic essential oil was toxic to T. molitorlarva, followed by pupa and adult. Diallyl disulfide was the most toxic than diallyl sulfide for pupa > larva > adult, respectively, and showing lethal effects at different time points. Garlic essential oil and their compounds have the potential for pest control [51]. The rosehip seed contained valuable phytochemicals such as phenolic compounds (2554 μg/g), carotenoids (2.92 μg/g), and ascorbic acid (1798 μg/g). Furthermore, the rosehip-seed oil was rich in polyunsaturated fatty acids, linoleic acid (54.05%), linolenic acid (19.37%), and phytosterols, mainly β-sitosterol (82.1%) [52]. Comparing the toxicity of the plant oils, it was affirmed that rose volatile oils was efficient natural phyto compounds against the treated larvae of cotton leaf-worm and combination of oils has synergistic action against the 4th larval instar of S. littoralis [53]. Rapeseed oil is plant-based oil extracted from the seeds of the rapeseed plants contained higher levels of total tocopherols and carotenoids. It is often added to animal feeds, when used in spray treatment against spider mites, green peach aphid and pear psylla resulted in efficacy rate 97.4% (7 days after treatment) against the summer population of P. ulmi, efficacy was 84.1% and 94.6%, 11 days after treatment against T. urticae and against M. persicae on pepper revealed high (> 96%, 7-14 days) efficacy of as and in a greenhouse achieved 86.9% efficacy after 8 days [54]. The synergistic action of essential oils mixture was also reported in antimicrobial activity [55-57]. Ag NPs (75 ppm) showed rapid 100% death at the 1st hour and no movement was observed till the 3rd hour. The result indicated highly and rabid effect of Ag NPs under the used concentration and particle size. Silver nanoparticles have a significant impact on insect antioxidant and detoxifying enzymes, leading to oxidative stress and cell death [58]. Ag nanoparticles also reduced acetylcholinesterase activity, while polystyrene nanoparticles inhibited CYP450 isoenzymes. Metal nanoparticles can bind to S and P in proteins and nucleic acids, respectively, leading to a decrease in membrane permeability. In the other hand, Quantitative proteomics studies on human colon cancer cell lines indicated that some cellular responses triggered by Ag NPs are driven by the size of NPs. The 100 nm NPs exerted indirect effects via serine/threonine kinase (PAK), mitogen-activated protein kinase (MAPK), and phosphatase 2A pathways, while the 20 nm NPs induced indirect effects on cellular stress, including generation of ROS, protein carbonylation and up-regulation of proteins [59]. The Origanumvulgare essential oil and silver nanoparticles have potent antibacterial activity [60]. MANPs (100 ppm) had slower acaricide effect starts with 40.66 ±0.49% at 1 h followed by 100% at 2 and 3 h. The slow killing activity of MANPs can be attributed to its very low toxicity which considered very safe environmental nanoparticles. The tested silver – essential oils nanocomposite showed acaricide effect 100% after 1, 2 and 3 h contact. This is the same obtained result from each compound alone. While, magnetite – essential oils nanocomposite showed 70.33±0.47% at 1 hour and 100% at 2 and 3 h so MANPs reduced the activity of essential oil.
Table 1 Acaricidal effect of different materials against D. gallinae collected from layers farms (average of 3 replicates)
|
Group |
Treatment |
Dose |
Mites mortality (%) |
||
|
1 h |
2 h |
3 h |
|||
|
1 |
Non treated |
Saline |
0 |
0 |
0 |
|
2 |
Oils |
0.4% |
100 |
100 |
100 |
|
3 |
MANPs |
100 ppm/mL |
40.66 ±0.49 |
100 |
100 |
|
4 |
Ag NPs |
75 ppm/mL |
100 |
100 |
100 |
|
6 |
Magnetite-essential oils nanocomposite |
1 mL |
70.33±0.47 |
100 |
100 |
|
6 |
Silver-essential oils nanocomposite |
1 mL |
100 |
100 |
100 |
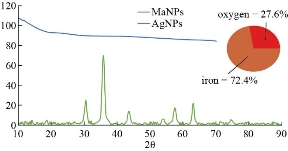
Fig. 1 XRD curves of MANPs and Ag NPs showing the amorphous state of Ag NPs and peaks of magnetite without any shift with 72.4% iron composition.
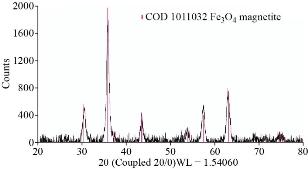
Fig. 2 The XRD pattern of MANPs – essential oil nanocomposite without any change in shift of magnetite peaks or change in lattice crystal.
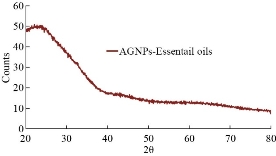
Fig. 3 XRD pattern illustrated the amorphous nature of Ag NPs – essential oil nanocomposite.
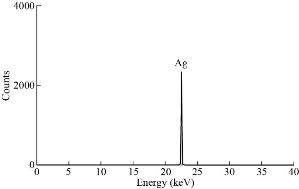
Fig. 4 XRF chart of Ag NPs.
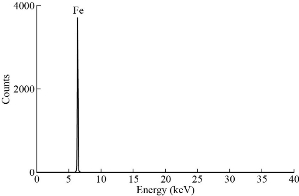
Fig. 5 XRF chart of MANPs.
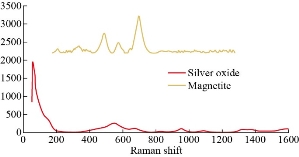
Fig. 6 Raman spectra of MANPs and Ag NPs.
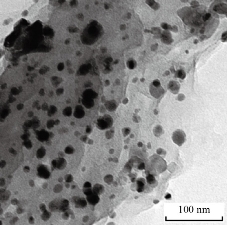
Fig. 7 TEM image of MANPs.
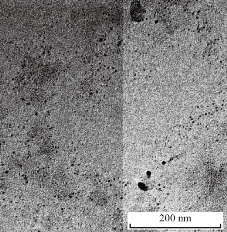
Fig. 8 TEM image of Ag NPs.
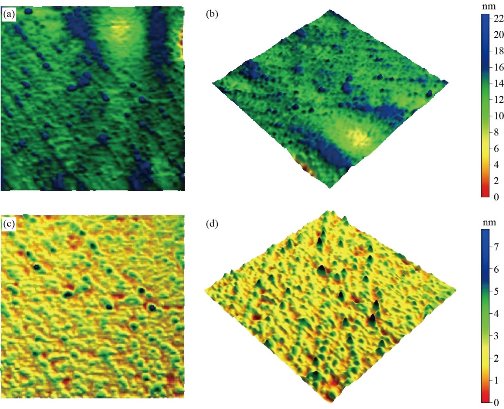
Fig. 9. (a) 2D AFM image for MANPs (top view 500 × 500 nm); (b) 3D AFM image for MANPs (500 × 500 nm); (c) 2D AFM image for Ag NPs (top view 500 × 500 nm); and (d) 3D AFM image for Ag NPs (500 × 500 nm).
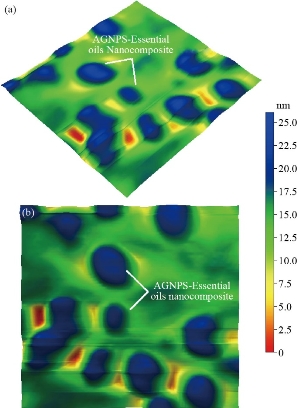
Fig. 10 (a) 3D AFM image for Ag NPs-essential oil (500 × 500 nm); (b) top view AFM image for Ag NPS-essential oil (500 × 500 nm).
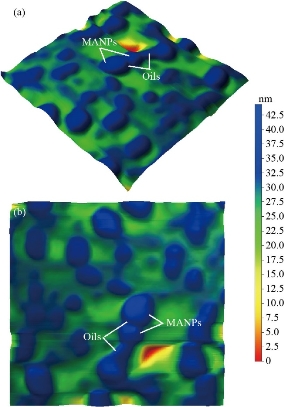
Fig. 11 (a) 3D AFM image for MANPs-essential oils (500 × 500 nm); (b) top view AFM image for MANPS-essential oils (500 × 500 nm).
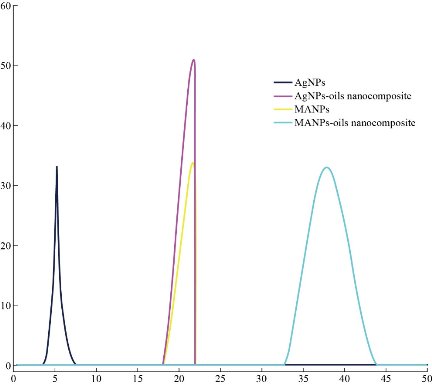
Fig. 12 Size of nanoparticles and nanocomposite using DLS.
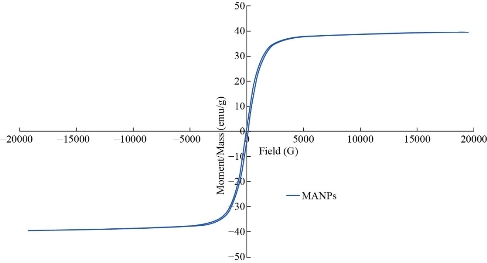
Fig. 13 The VSM results of MANPs.
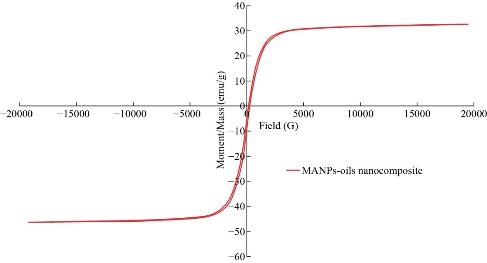
Fig. 14 The VSM results of MANPs – essential oil nanocomposite.
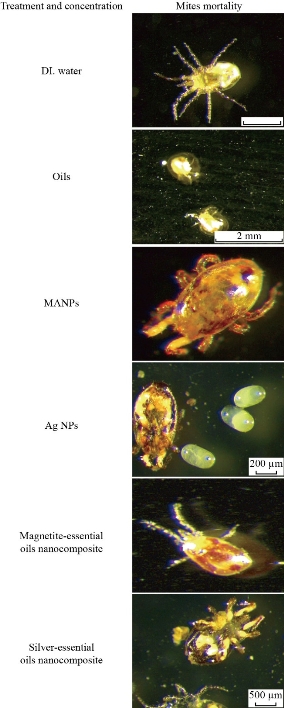
Fig. 15 Photos of acaricidal effect of essential oil and nanoparticles against D. gallinae collected from layers farms.
Conclusions
Silver nanoparticles, magnetite nanoparticles, silver-essential oil nanocomposite and magnetite-essential oil nanocomposite were synthesized and their morphological, identification and index properties were characterized. According to killing time and effect on D. gallinae bodies, the highest killing activity was shown by silver nanoparticles, followed by silver-essential oil nanocomposites, essential oil, magnetite nanoparticles, and lastly magnetite-essential oil nanocomposites. Apart from that, silver-essential oil nanocomposites and silver nanoparticles presented 100% killing activity after a few seconds, while essential oil, magnetite-essential oil nanocomposites and magnetite nanoparticles showed 100% killing activity after 1 to 2 h. If not considering the time of killing, magnetite-essential oil nanocomposites were more recommended to be used than silver-essential oil nanocomposites and silver nanoparticles due to their very low toxicity. However, if taking time into account, silver-essential oil nanocomposite and silver nanoparticles could be beneficial and involved in D. gallinae (red mite) control strategies in poultry industry.
Acknowledgments
Synthesis, characterization and observed killing activity of nanoparticles and nanocomposites carried out in Egyptian Nanotechnology Center – Cairo university (EGNC).
Funding
The authors declare that the work was self-funded.
Authors’ contributions
Aziza M. Amer and Mohamed M. Amer designed, planned the study samples and all laboratory tests. Sameh H. Ismail and Gehad G. Mohamed synthesized and characterized nanoparticles and nanocomposite. All authors shared performed experimental work, manuscript writing, drafted, revised the manuscript and approved the final manuscript.
Conflict of Interests
The authors have no conflict of interests to declare regarding the publication of this paper.
References
Copyright© Sameh Ismail, Gehad Mohamed, Aziza Amer, and Mohamed Amer. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.