Research Article
Development and Head-to-Head Comparison of Two Colloidal gold Based Serologic Lateral Flow Assays for SARS-CoV-2 Antibody Tests
Jing Tian 1, Kan Wang 2, Yanlei Liu 2, Hui liang 1, Xueling Li 1, Daxiang Cui 1,2 *
1 National Engineering Research Center for Nanotechnology, Shanghai, China.
2 Institute of Nano Biomedicine and Engineering, Shanghai Engineering Research Centre for Intelligent Diagnosis and Treatment Instrument, Department of Instrument Science and Engineering, School of Electronic Information and Electrical Engineering, Shanghai Jiao Tong University, Shanghai, China.
* Corresponding authors. E-mail: dxcui@sjtu.edu.cn
Received: Aug. 25, 2020; Accepted: Aug. 31, 2020; Published: Oct. 25, 2020
Citation: Jing Tian, Wang Kan, Liu Yanlei, Liang Hui, Li Xueling, and Cui Daxiang, Development and Head-to-Head Comparison of Two Colloidal Gold Based Serologic Lateral Flow Assays for SARS-CoV-2 Antibody Tests. Nano Biomed. Eng., 2020, 12(4): 306-310.
DOI: 10.5101/nbe.v12i4.p306-310.
Abstract
To combat the COVID-19 pandemic, serologic lateral flow immunoassays are required to facilitate accurate diagnosis of SARS-CoV-2 infection and confirmation of molecular results. This study evaluated sensitivity of two different designs of colloidal gold serologic tests (antigen based total antibody test and antibody based IgG test) by using residual serum samples from patients who were evaluated for SARS-CoV-2 infection status by polymerase chain reaction (PCR). The results showed 100% specificity for both tests, while when testing of 16 positive patients, the data showed 90% sensitivity for total antibody test and 30% for IgG test. This study demonstrates high diagnostic accuracy for anti-SARS-CoV-2 total antibody tests and will facilitate further development and selection of serological assays.
Keywords: SARS-CoV-2, COVID-19, Rapid point-of-care tests (POCTs), Serologic lateral flow assays (LFA), Colloidal gold
Introduction
A new coronavirus, 2019-nCoV, has recently emerged to cause a human pandemic. In February 2020, 2019-nCoV is officially classified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. The World Health Organization (WHO) announced that the official name of the disease caused by this virus is Corona Virus Disease (COVID-19). As of August 20th 2020, the virus has infected over 22,263,000 individuals and caused 784,107 deaths worldwide. SARS-CoV-2 is the seventh coronavirus known to infect humans and can cause severe disease as SARS-CoV and MERS-CoV. The SARS-CoV-2 genome is a single-strand positive sense RNA of 29,903 nucleotides. SARS-CoV-2 has four major structural proteins: the envelope spike protein S, the small envelope protein E , the matrix protein M, and the nucleocapsid protein N [2,3,4]. Spike protein S mediates attachment to cellular receptors and entry by fusion with cell membranes. Nucleocapsid protein N is the most abundant protein of coronavirus, which binds to viral RNA and leads to formation of the helical nucleocapsid. Both S and N are major antigen, which have been shown to elicit remarkable IgG and IgM responses, and both of them are frequently used as diagnostic candidates [5, 6].
RT-PCR is the standard and recommended method for the diagnosis of SARS-CoV-2 , but the limitation of this technology is obvious, like long turnaround time, requirements for certified laboratories and skilled technicians etc. Serological testing, on the other hand, is easy to perform and helpful for the diagnosis of suspected patients with negative RT-PCR results and for the identification of asymptomatic infections. But serologic testing such as lateral flow immunoassays (LFAs) can have wide performance range based on the viral antigens used, how they were designed and developed, also the construction of the cassette. Based on these, we compared two different designs of colloidal gold LFAs and developed an antigen based LFA test product, which can detect total antibody to SARS-COV-2 in human blood within 15 minutes with high sensitivity.
Experimental
Materials
Goat anti-human IgG (ab97221) and rabbit anti-goat IgG antibody(ab6741) were purchased from Abcam. Normal goat IgG control (CR2), SARS-CoV-2 Spike antibody (rabbit polyclonal antibody, 40591-T62), and SARS-CoV-2 Spike Protein (40592-V05H) were developed and purified at Sino Biological. Bovine serum albumin (BSA) was obtained from sigma. Potassium carbonate, Sucrose, Sodium tetraborate, Orthoboric acid were obtained from Damas-beta. 40nm colloidal gold solution, NC membrane, the glass fiber and PVC pads were obtained from Shanghai JN Bio Inc.
Preparation of colloidal gold conjugates
Goat anti-human IgG conjugates and normal goat IgG control conjugates
To 10 mL of colloidal gold was added 200 µl of 1% K2CO3 and then 100 µl of 1.0 mg/mL antibody (goat anti-human IgG or normal Goat IgG control) was added to the colloidal gold solution. The whole mixture was stirred for 30 minutes at room temperature. Then, 1mL of 10% BSA was added to the solution to block the surface of gold nanoparticles. After incubation at room temperature for 15 minutes, the solution was centrifuged at 8000 rpm for 20 minutes. The supernatant was removed, and 10mL of 1% BSA in 20mM boric buffer (pH8.0) was added to resuspend the pallet. Repeat the above steps twice, the pallet was resuspended in 200µl of colloidal gold storage buffer and stored at 4 °C.
Spike protein conjugates
150 µl of 1% K2CO3 was added to 10 mL of colloidal gold to adjust the pH and then 40 µl of 2.0 mg/mL spike protein antigen was added to the solution. The following steps are the same except that 1% BSA in 20mM boric buffer (pH9.0) was used as the resuspend solution.
Preparation of test cassettes
The nitrocellulose membrane was coated with recombinant SARS-CoV-2 spike protein at the T (test) line, and rabbit anti-goat IgG antibody at the C (control) line. NC membrane was then dried for 2 hours at 37 °C. To prepare antigen conjugate pad, spike protein and normal Goat IgG control conjugates were diluted 20 times with dilution buffer, mix the two solutions with 4:1 ratio and apply to the glass fiber pad. The glass fiber pad was then dried for 2 hours at 37 °C. To prepare antibody conjugate pad, the goat anti-human IgG conjugates were diluted 20 times with dilution buffer and apply to the glass fiber pad. The glass fiber pad was also dried for 2 hours at 37 °C. The test strip consists of five parts, including plastic backing, sample pad, conjugate pad, absorbent pad and NC membrane. The sample pad overlayed the conjugate pad by 1-2 mm, the nitrocellulose membrane was overlayed by the conjugate pad (1-2 mm). Finally, at the distal side, the nitrocellulose membrane was overlaid by the absorbance pad. The membrane set was then assembled into a plastic cassette and packaged in an aluminum pouch with a silica desiccant bag inside to keep the humidity low (Fig. 1).
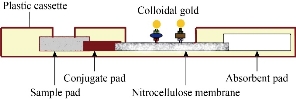
Fig. 1 Side view of a lateral flow immunoassay.
SARS-CoV-2 specimens
The sensitivity of the POCTs was evaluated with 16 samples from convalescent plasma donors. These individuals had been diagnosed as RT-PCR positive for SARS-CoV-2, and asymptomatic for at least 28 days.
Serologic assays
The tests were performed at the sites by clinical staffs with following test procedure. Briefly, add 10µL serum/plasma specimen into each specimen well. Add 80µL or 2 drops of specimen diluent into each specimen well. Wait for the colored line(s) to appear. Any detectable band at the T line was considered as a positive result. Results were considered invalid when the control band was not visible.
Analysis
Specificity and sensitivity was calculated for total antibody test and IgG test separately using the following equation: Specificity (%) = 100 x [True negative / (True Negative + False Positive)]. Sensitivity (%) = 100 x [Positive/ (Positive + False Negative)].
Results and Discussion
The SARS-CoV-2 antibody based IgG test is designed as showed in Fig.2. Briefly, antibody conjugate pad was used to provide colloidal gold conjugated goat anti-human IgG antibody as a tracer. The SARS-CoV-2 S antigen was coated at T line of the NC membrane and rabbit anti-goat IgG antibody at the C line. After addition of the specimens onto the sample pad, colloidal gold-labeled goat-anti human IgG antibodies would be released and bind with human IgG in serum. If any anti- SARS-CoV-2 IgG was present, it would be captured at the T line. The binding of colloidal gold-labeled goat-anti human IgG antibodies with rabbit-anti-goat IgG antibodies would form a visible purple line as the control line(C), which means the system works fine. For Antigen based total antibody test, all other components are the same except that antigen conjugate pad was used instead (Fig.3). Their NC membrane settings are the same too, S antigen coated at T line and rabbit anti-goat IgG antibody at the C line. When the specimen was applied onto the sample pad, colloidal gold-labeled S antigen would be released and bind with anti- SARS-CoV-2 total antibody, mainly IgG and IgM, and was further captured by pre-coated SARS-CoV-2 S antigen, and generate a purple line at T (Fig. 3). The specificity and sensitivity of the two different tests were evaluated clinically with the RT-PCR positive serum and negative serum. A total of 26 positive cases were tested: 16 (positive) clinically confirmed (PCR test) SARS-CoV-2-infected patients and 10 negative patients. The testing results were summarized in the Table 1. Of the 16 positive samples, 12 tested positive for total antibody test, resulting in a sensitivity of 75.0% (Fig.5). While using the same 16 positive samples, only 6 tested positive for IgG test, resulting in a sensitivity of 37.5% (Fig. 4). All negative samples were tested as negative for both total antibody and IgG tests, which means the specificity of both tests are 100%. (Table 1). As of May 18th 2020, 44 SARS-CoV-2 detection kits and equipment were granted through Chinses Food and Drug Administration (FDA) emergency use authorization. 18 of them are serologic immunoassays and 16 are IgM or IgG only tests. Researchers have noticed that Rapid point-of-care tests (POCTs) for SARS-CoV-2-specific antibodies vary in performance. Steven E Conklin et al., reported that 15 POCTs evaluated had a combined sensitivity and specificity for IgM and IgG between 55%-97% and 80%-100%, respectively. When assessing the performance of the IgM and the IgG bands alone, sensitivity and specificity ranged from 0%-88% and 80%-100% [8, 9]. The different performance between the assays may, in part, be explained by the assay format used. Specific antibody to SARS-COV-2 represent only a small part of antibodies in serum. Both specific antibody and non-specific antibody in serum are able to bind with colloidal gold-labeled goat anti-human IgG. The competition binding of non-specific antibody to gold conjugate would greatly decrease the sensitivity of the IgG test and lead to false negative. While, only specific antibody would bind to colloidal gold-labeled S antigen. Furthermore, total antibody test targets the IgA, IgG, and IgM. All these factors may explain why the antigen-based total antibody test showed better performance on sensitivity than the antibody based IgG test. A major limitation of this study was the low number of patients used for validation. In addition, commercial IgG kits should be included and evaluated together. Further studies will carry out later.
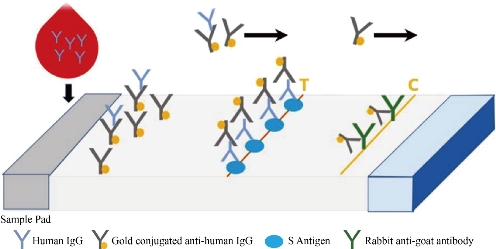
Fig. 2 Antibody based IgG test.
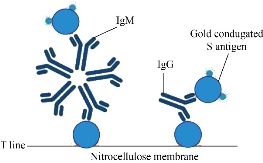
Fig. 3 Antigen based total antibody test.
Fig. 4 Sensitivity of antibody based IgG test.
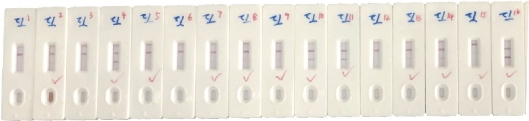
Fig. 5 Sensitivity of antigen based total antibody test.
Table 1 comparison between total antibody test and IgG test Please add table caption here
|
|
Positive |
Sensitivity |
Negative |
Specificity |
|
Total antibody test |
12 |
75.0% |
10 |
100% |
|
IgG test |
6 |
37.5% |
10 |
100% |
|
Total sample tested |
16 |
-- |
10 |
-- |
Conclusions
We compared two different designs of colloidal gold serologic tests, antibody based IgG test and antigen based total antibody test. The head to head evaluation of their clinical efficacy showed that the antigen based total antibody test had sensitivity of 75.0% while the IgG test showed sensitivity of 37.5%. The total antibody test we developed showed higher sensitivity than commonly used IgG test and would provide a more accurate SARS-CoV-2 infection diagnosis product.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Jing Tian, Wang Kan, Liu Yanlei, Liang Hui, Li Xueling, and Cui Daxiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.