Review
Green Synthesis of Silver Nanoparticles: An Eco-Friendly Approach
Sopan Namdev Nangare 1, Pravin Onkar Patil 1*
1 Department of Pharmaceutical Chemistry, H. R. Patel Institute of Pharmaceutical Education and Research, Shirpur-425405, (MS) India.
* Corresponding authors. E-mail: rxpatilpravin@yahoo.co.in
Received: Apr. 1, 2020; Accepted: Jul. 21, 2020; Published: Oct. 19, 2020
Citation: Sopan Namdev Nangare, Pravin Onkar Patil, Green Synthesis of Silver Nanoparticles: An Eco-Friendly Approach. Nano Biomed. Eng., 2020, 12(4): 281-296.
DOI: 10.5101/nbe.v12i4.p281-296.

Sopan Namdev Nangare is currently a senior research fellow (Indian Council of Medical Research) at HRPIPER, Shirpur. He has completed his M. Pharm from Bharati Vidyapeeth College of Pharmacy, Kolhapur. He has presented many research papers at various national and international conferences and symposiums. He has been awarded with “Best Outgoing Student” by Bharati Vidyapeeth College of Pharmacy, Kolhapur. He has more than 15 research papers and reviews articles published in national and international journals of repute. His research focuses on the green synthesis of nanocomposites, biosensing, natural polymer-carriers for drug delivery, etc.

Dr. Pravin Onkar Patil received his Ph.D. in January 2014 from R. C. Patel Institute of Pharmaceutical Education & Research, Shirpur (M.S.). He did his M. Pharmacy in Pharmaceutical Chemistry from NDMVP͛s College of Pharmacy, Nashik (2005). Presently, he is head of the Department of Pharmaceutical Chemistry at HRPIPER, Shirpur, and approved Associate Professor as well as PG teacher in Pharmaceutical Chemistry by North Maharashtra University, Jalgaon. He has presented papers in various conferences, published articles in national and international journals of repute. Recently, he obtained the research grant from SERB (DST), NMU Jalgaon, ICMR, etc. His major fields of scientific interest is green synthesis of graphene-based material for biosensing platform and novel pharmacophore development for several cancer targets using computational tools and their evaluation against a panel of human cancer cell lines using various in vitro assay techniques.
Abstract
Eco-friendly synthesis of nanoparticles is an upcoming discipline of nanoscience. Green synthesis of Ag NPs has gained immense importance and much awareness in developed nations. Fascinatingly, such an environmental friendly synthesis of Ag NPs gives a green chemistry-based non-toxic and economical route to nanotechnology. This review article gives insight into the bioinspired synthesis of Ag NPs and mechanisms involved in the synthesis of Ag NPs. In this review, we have summarized the scientific reports in the eco-friendly synthesis arena of Ag NPs and their applications in the biomedical field. Especially, we have focused on plant materials, fungi, algae, and bacterial potential towards the eco-friendly synthesis of Ag NPs. For future perception, there is a need for in silico and in vitro, in vivo research to authenticate the outcomes.
Keywords: Silver nanoparticles, Green synthesis, Plant extract, Eco-friendly, Nanotechnology
Introduction
Initially, Richard Feynman (American physicist) has reported the term ‘Nano-science’. Principally, it is the pioneering division of science, essentially focused on nonmaterial fabrication and its properties [1]. To date, nano-science opens the new route of “nanotechnology”, which is a promising and multidisciplinary arena; it covers the diverse field together with biology, engineering, chemistry, and physics, etc. Primarily, it is the branch of nano-science, which includes the synthesis, manipulation, and use of nanoparticles (NPs, at least one dimension in the range of 1-100 nm) [2, 3]. In contrast to the macro-particles, it demonstrates noteworthy modification in properties, generally chemical, physical, and biological properties. At present, increase multiple drug resistance microorganisms (example: bacteria, fungi, virus, etc.), health-hazardous active pharmaceutical ingredients, and poor dietary intake crisis. Owing to that, from the last few years, it enhanced the microbial challenges for humans and the environment. For overcoming these challenges, a researcher focused on the innovative sector of nanotechnology, which resolves the troubles of multiple drug-resistant microorganisms and enhanced the rate of metabolism. In addition, it creates a pioneering pathway for the investigation, which explores the precise alternative to treat and resolve the key challenges of microorganisms [3]. Abundant literature reported that the diverse types of NPs including carbon NPs, polymer-based NPs, lipid-based NPs, magnetic NPs, metal oxide NPs, and metal nanoparticles (MNPs) used in the field of nanotechnology [4-6]. Besides, the MNPs give exceptional attractive optical and electronic properties, which are related to the quantum size effect. Moreover, it gains promising applications in the pasture of optics and catalysis to chemical/biochemical sensing [7]. On the other hand, fields of electronics, magnetic, optoelectronics, and information storage are shown an extra awareness towards the noble NPs, such as gold NPs (Au NPs), silver NPs (Ag NPs), platinum NPs (Pt NPs), palladium nanoparticles (Pd NPs).
Silver Nanoparticles
From ancient times, silver metal is frequently used in the management of a range of ailments [8]. Since, beginning of past decades, nanotechnology has focused on the utilization of silver by nano-engineering to NPs in special fields [9] viz. photonics [10], micro-electronicsm [11], medicine [12, 13], etc. The most ordinary use of Ag NPs in biomedical applications is accredited to their antimicrobial activity against a broad variety of microorganisms [14, 15]. Moreover, physical and chemical techniques viz. laser ablation [16], lithography [17], photochemical reduction [18], etc. taking the account for synthesis of Ag NPs. In addition, various Ag NPs synthesis approach has been reported in nanoscience. Furthermore, the various scientific reports revealed the two divisions for the synthesis of the Ag NPs include top-down and the bottom-up approach. Briefly, the top-down approach for the synthesis of Ag NPs includes cutting, milling, etching, thermal decomposition method, lithography, and laser beam processing, etc [19]. A further approach is a bottom-up approach, which comprised green synthesis, spinning, plasma spray synthesis, sol-gel process, supercritical fluid synthesis, etc. [20].
Green Synthesis of Ag NPs
The invention of NPs using previously reported methods having the major drawbacks, especially mass production, chemical residual, cost, and toxicity, etc. and to conquer problems such as the green synthesis route of Ag NPs gaining more attention of the researcher [14, 21]. The green synthesis approach for NPs synthesis is a more superior, facile, eco-friendly, biocompatible, biodegradable, non-toxic, and cost-effective [22-24]. It is base on a variety of green sources (example: plant extract, polymers, starch, pectin, proteins, amino acids, bacteria, fungi, food-waste, agro-waste, microbes, bacteria, virus, biopolymers) which stabilize/capped or reduce or both the NPs [14]. The synthesis scheme of Ag NPs by plant extract, micro-organism, etc. depicted in Fig. 1. In the earliest days, the plants and their derivatives were widely used in the medical applications, and this plentiful potential of the medicinal plant have been used in nanotechnology to generate the subsequent generation of nano-pharmaceuticals [25]. Some biological methods have been employed to produce NPs without any toxic, harsh, and expensive substances. In addition, some reports claimed the use of microorganisms [26, 27] is readily scalable, eco-friendly, and compatible with biomedical applications [28, 29].
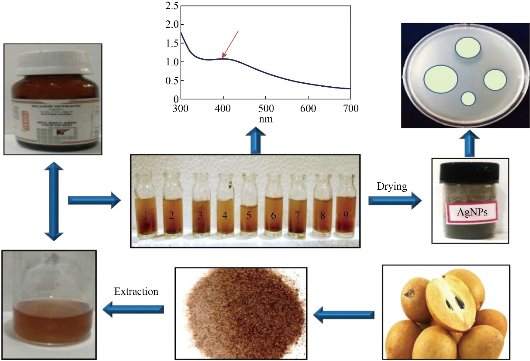
Fig. 1 Bioinspired synthesis of Ag NPs.
Mechanism Involved in the Synthesis of Ag NPs
The recent studies have predicted possible Ag NPs synthesis mechanisms. The polyphenols, proteins, amino acids, enzymes, etc. plays a crucial role in reducing of Ag+ ions, which is commonly present in plant extract, agro-waste, microorganism, etc. Most of the investigations claimed that the phenolic compounds, proteins, amino acids, etc. possess hydroxyl, carbonyl functionalities that could inactivate silver ions by chelation. The chelation properties of such actives can be correlated with their high nucleophilicity. Herein, the schematic representation for the mechanism involved in the synthesis of Ag NPs has shown in Fig. 2. The present review is focused on the facile, eco-friendly, biocompatible, cost-effective green synthesis of Ag NPs. In addition, it gives an insight into variousgreen sources, including plant extract, agro-waste, food-waste, fungi, microbes used for the synthesis of Ag NPs along with its anti-microbial, antifungal activity against a different type of pathogenic microorganism and its possible mechanism of action. It provides a brief insight into the biomedical application of Ag NPs. Additionally, here we notice the various quality parameters, which affect the quality, stability as well as the particle size of NPs. Owing to this; we endeavor to review the various quality attributes that play a crucial role in the synthesis of Ag NPs.
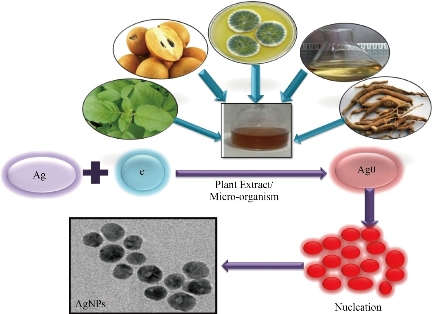
Fig. 2 Mechanism involved in the synthesis of Ag NPs.
Plant Extracts as a Reducing Agent
Plants are a huge source of active phytoconstituents and also known as the immortality origin. The use of such actives in the treatment of various ailments viz. cough, hay asthma coryza, bowel complaints, and bronchial infections, etc. are an excellent alternative to exiting problems arise from synthetic ones. Literature survey evinced that the Moringa oleifera (Moringaceae) leaves extract used for synthesis of Ag NPs provides the natural healing properties, high antioxidant properties, as well as antibacterial activity against gram-positive or gram-negative bacteria. Recently, leaf extract was used for the Ag NPs synthesis. It showed a direct and complete reduction of Ag+ in sunlight. Consequently, it observed that the biosynthesized Ag NPs offers the broad-spectrum antimicrobial susceptibility range. Therefore, it could release a new substitute for biomedical purposes [30]. Antifungal agent, fluconazole (FLZ, azoles agent) used for the treatment of fungal infections. But, the emergence of FLZ-resistant candid, gives limitation for the use of FLZ. Thus, the present work investigated the Ag NPs by green route using plant extracts of ginger (Zingiber officinale) and thyme (Thymus vulgaris). It gives reduced and coated Ag NPs. These Ag NPs showed the antifungal activity against Candida albicans along with that it free from cytotoxicity below 3.5 ppm concentration which is more superior to the FLZ. Thus, it could be served as a potential antifungal agent instead of FLZ for the treatment of superficial fungal infections [31]. The eco-friendly synthesis process raises the attention of the researchers in the nanoscience arena. Owing to that the Shaik and co-authors prepared the Ag NPs by using the Saudi Origanum vulgare (O. Vulgare) L. extract as a reducing agent for silver ions. It has been claimed that the capping capacity of extract containing actives offered the stabilization of Ag NPs. Besides, these prepared NPs were checked for the antibacterial and antifungal activity including Shigella sonnei, Micrococcus luteus, E. coli, Alternaria alternate, Paecilomyces variotii, Aspergillus flavus, Phialophora alba, etc. pathogens. It gives the uppermost activity against P. aeruginosa (Gram-negative strain), S. aureus (Gram-positive strain) amongst the variety of bacterial strains tested. Also, it has shown for more effectivity against Alternaria alternate fungal species [32]. The earlier day’s researcher focused on the cost-effective and eco-friendly synthesis of nanoparticles. This anticipated work investigated the use of Oryza sativa husk extract as a reducing agent for Ag NPs preparation. It formed the silver hydrosol because Ag ions possessed good conductivity, chemical stability, and catalytic activity. Moreover, it has been reported that these prepared NPs provide the antibacterial activity against P. aeruginosa and S. aureus. In conclusion, Ag NPs showed the excellent ZOI for P. aeruginosa as a contrast to S. aureus concerning Amikacin (standard) [33]. In a medical and industrial process, silver proves a very important role due to it’s an inhibitory effect on microbes present in the medical and industrial process. Additionally, Ag NPs have more application and utilization in the topical ointment to the treatment of wound and infection. Especially, for those wound and infection are caused by burning. The present report prepared Ag NPs by using Argemone maxicana leaf extract as a reducing and capping agent for silver ions. Furthermore, it showed the antibacterial activity against gram-positive and gram-negative bacteria due to the bactericidal effect Ag+ of along with that its membrane-disrupting upshot of the polymer subunit. Thus, considering the outcomes of the investigation, it could be more constructive in the topical application [34]. To overcome the drawbacks of the chemical method, the researcher focused on the new eco-friendly method for the synthesis of Ag NPs. Recently, Alpinia katsumadai seed extract was used as a capping and reducing agent for the synthesis of Ag NPs. This seed extract contains proteins and flavonoids, which furnished the reduction of Ag+ to Ag0 ion. Owing to the presence of amine and carbonyl functional groups, Ag NPs capped and further stabilized. Besides this, it provides excellent free radical scavenging activity (by DPPH method). In this way, it showed potent antibacterial activity against bacteria. Thus it is an eco-friendly method for NPs and in prospects, thus it could be used in the management of various ailments [35]. Anciently, silver plays a crucial role in the treatment of common ailments including infection in burns, open wounds, and chronic ulcers, etc. Nowadays, antibiotic resistance is a key crisis reported by various investigators and it could be conquered by using nanoscience techniques. Also, antibacterial clothing, coating for the medicinal device, burn ointments, etc. are the challenges for researchers. Thus there is a need to investigate the suitable alternative to conquer all crises. Krithiga and investigators reported the green synthesis of Ag NPs using leaf extract of Clitoria ternatea and Solanum nigrum by manipulating the optimization parameters. Due to the existence of carboxyl, amine, and hydroxyl group, the MNPs of silver was successfully synthesized. After that, these NPs checked for antibacterial activity. As a result, it exhibited an excellent antibacterial effect against nosocomial pathogens [36]. The new global era of science having drug resistance is a major deadlock towards the developments. Owing to that, the multidrug resistance is a major challengeable path for a scientist. Now, there is a need to develop the new technology and formulation for overcoming this type of confines. According to the various scientific investigations, natural sources have an enormous potential to conquer such drawbacks and crises. Reported work investigated the use of Sonneratia apetala Buch.-Ham. (S. apetala) leaves in the synthesis of Ag NPs. These prepared NPs were revealed the high antioxidant and antibacterial activities. Consequently, the eco-friendly method provides a wide potential for against Proteus mirabilis pathogen [37]. Another report revealed the use of green source plant leaf (Hibiscus leaf) in the preparation of Ag NPs. The increase in temperature rate, the reactant consumption rate is high and due to that the particle size of NPs reduces. Also, increased heating time boosts NPs particle size. Moreover, Ag NPs size has been increased by the increased concentration of silver nitrate [38]. Roheda (Tecomella undulata G. Don, Bignoniaceae) a well-known medicinal plant, contains huge medicinal value towards the spleen, liver, and abdominal complaint. Since, due to the presence of flavonoids, glycosides, alkaloids, saponins, etc. it provides the antibacterial, antioxidant anti-inflammatory, analgesic potential, and hepatoprotective activity. The present investigation revealed the use of flower extract of roheda in the synthesis of Ag NPs. Owing to the existence of a C-H bond, C-O bond, and C-N bond/ stretching vibrations, it stabilized and reduced the silver ions into NPs [39]. Shekhawat and co-authors have reported the use of Cardiospermum halicacabum (C. halicacabum) leaf aqueous extract as a reducing agent for syntheisis of Ag NPs. Also, it has been reported that the carboxyl functional groups in aspartic or glutamine residues, and hydroxyl groups in tyrosine residues of the proteins, reduced the silver ions and stabilized the Ag NPs [40]. Raspberry plant has been majorly used as an antiseptic, anti-leucorrheal, anti-abortient, anti-gonorrheal, and anti-malarial. Recently authors used the aqueous extract of raspberry leaf for green and eco-friendly synthesis of Ag NPs. Due to the potential of inhibitory action of Ag NPs on microorganisms, it showed an excellent antibacterial activity. The probable antibacterial mechanism is based on the interaction among silver ions and thiol groups of the essential enzymes of bacteria and due to these, NPs inactivate the microorganism. The interaction at the very initial stage, Ag NPs adhered to the bacterial cell wall and the surface charge of the functional group of Ag NPs upset the permeability and respiration functions of the cell and due to that, cell viability is getting diminish and it resulting into cell death[41]. The successful attempt of green and cost-effective synthesis of Ag NPs has been made using seed extracts of Cyperus esculentus (C. esculentus) and Butyrospermum paradoxum (B. paradoxum). The phenolic compounds possess hydroxyl and carboxyl groups.The high phenolic content contains plants that are the best candidates for NPs synthesis[42]. The Glycyrrhiza glabra L. (G. glabra, Fabaceae) is a medicinal plant and widely used in ulcer treatment. The present investigation reported the G. glabra L. root extract having high reduction and stabilizing ability towards the Ag NPs. Also, Owing to the existence of biomolecules observed in extracts mainly enzymes or proteins, polysaccharides, amino acid, and vitamins, the Ag+ ions reduced into the Ag NPs [43]. According to the recent scenario of nanotechnology, plant extracts have been widely used as original and cheap reducing agents to fabricate NPs for broad purposes. This study revealed the utilization of the aqueous extract of Pimpinella anisum (P. anisum) seeds in the synthesis of Ag NPs. The seed extract showed the reduction and stabilizing aptitude of Ag NPs. Moreover, it showed the enormous antibacterial potential for gram-negative and positive bacteria. In addition, it provides good cytotoxicity on hSSCs and HT115s cells. Accordingly, it could be used for cancer treatment and various biomedical applications [44]. Nowadays, the waste to wealth based green synthesis of Ag NPs approach skips the obligation of costly instruments, hazardous chemicals. This study demonstrated the use of leaf extract of rosemary in the synthesis of Ag NPs. It showed a non-significant inhibitory upshot on the germination percentage of wheat, pigment fractions, and dry weight. Furthermore, it was observed that the biosynthesized Ag NPs has a clear stress cause on tomato plant as reduced chlorophyll and dry weight. It has been reported that the Ag NPs stimulate malondialdehyde accumulation in tomato and wheat plants. Thus, it has been concluded that Ag NPs offered both effect (positive and negative) on wheat seedling and vegetative growth of the plant (wheat and tomato)[45]. The present investigation was used the leaf extract of A. andrachne for the fabrication of Ag NPs. The FTIR study reveals the confirmation of the binding of Ag NPs to the oxygen of the hydroxyl group of phyto-constituents. Furthermore, it showed the antibacterial effect against B. cereus, S. aureus, E. coli, K. pneumoniae, P. aeruginosa, Candida albicans, etc [46]. The bioinspired synthesis of Ag NPs was carried out by using fruit extract of Coccinia grandis (C. grandis) and Phyllanthus emblica (P. emblica) as reducing and stabilizing agent. The evolutionary study including primarily FTIR showed the peaks for the functional group (N–H bond amines) which confirmed the presence of reducing and stabilizing agents, and due to that the conversion of silver ions to Ag NPs exists. Ag NPs of C. grandis showed the maximum antibacterial activity against V. cholera. Whereas, Ag NPs from P. emblica gives maximum inhibition against S. aureus, V. cholerae, and S. typhi, as well as Proteus mirabilis [47]. A tropical plant, Sapota Pomace (Manilkara zapota) fruit contains high phenolic, dietary fiber content. Also, the pomace (waste residue), provides huge phenolic and fiber content. A pomace containing functional group including hydroxyl and alkyne C-H bond gives the confirmation of phenolic and amine group (protein), which showed the reducing and capping for silver ions. In addition, it provides the antibacterial effect against gram-positive and negative bacteria [48]. Extract of neem, tea (Camellia sinensis), and peels (kitchen waste of tomato, carrot, snake sweet potato, pumpkin, mango, and drumstick) were successfully used for the synthesis of Ag NPs. The FTIR study reveals the presence of flavonoids in extract and these flavonoids containing hydroxyl and amino groups give the stability for silver and reduce the Ag+ to Ag0. Also, prepared NPs were subjected to the antibacterial activity and it showed the antibacterial effect against gram-negative as well as gram-positive bacteria. Also, it gives the reportable antioxidant activity[49]. Yet another study reported that the use of mint leaf extract for the synthesis of Ag NPs, along with that the authors were claimed the possible mechanism for antibacterial activity on E. coli and B. subtilis. The NPs of silver ions may interact with the cell membrane and due that the phosphorus-based compound (DNA) and replication process may get hamper and because of this the cell loses their viability and ultimately the cell death occurs. Also, AgNP's effect on the respiratory function of bacteria, as well as silver can interact with a thiol group enzymes and as a result inactive the cell and its function [50]. One more report claimed the synthesis of Ag NPs using a green route. Briefly, Satin leaf (Chrysophyllum oliviforme, Sapotaceae) extract was showed a strong reducing capacity of silver ions. The FTIR study confirmed the, –OH/>N-H and >C=N groups in the extract, which are responsible for the reduction of silver nitrate to nano-silver. The result of antioxidant and anticancer activity showed that the NPs having potent nature towards the various pathogens [51]. Since past decades the green synthesis grabs the more attention of researchers. These NPs showed outstanding advantages including a greater surface area to volume ratio and highly reactive than macromolecules. Krishnadhas and coauthors were reported that the use of fresh leaves of Volkameria inermis extracts as a reducing agent for Ag NPs. The FTIR study revealed the existence of hydroxyl, alkene, aldehyde, etc., All these actives capped and stabilize the Ag NPs[52]. Recently, Eriobotrya japonica (Thunb.) leaf extract showed the potential for the synthesis of Ag NPs and it showed the stabilizing and reducing ability for silver ions into NPs. Authors were claimed that the higher the synthesis temperature, the larger particle size and ultimately slower the reaction rate [53]. The victorious endeavor for green synthesis of Ag NPs has been made using stem latex of Jatropha curcas. The outcome of the investigation concludes that the presence of -NH and C=O group of cyclic peptides (curcacycline A and curcacycline B) synthesized the NPs of silver. In addition, these actives capped the Ag NPs along with reduction of the silver ions from Ag+ to Ag0 [54]. In 2013, Kotakadi and co-authors synthesized the Ag NPs using dried leaves of Catharanthus roseus. Linn. G. Donn (Vinca rosea). The particle size, zeta potential of Ag NPs was found to be 27 nm to 32 nm and −63.1 mV respectively. The high negative zeta potential confirms the repulsion among Ag NPs and thereby it showed admirable stability. Additionally, the energy dispersive X-ray showed the presence of elements, which confirmed the reduction of Ag+ to Ag0. It has been reported that the synthesized Ag NPs (10μL) showed higher antibacterial activity against Pseudomonas fluorescens, and intermediated activity against E. coli, S. aureus, and lactobacillus using amoxyclav as a reference. To sum up, this can be a new avenue for numerous biomedical applications [55]. Another study reported that the eco-friendly, economical, and cost-effective synthesis of Ag NPs using plant extract of Andrographis paniculata (Nelavemu) that exhibited the well-defined absorption peak at 433 nm. The concentration of functional groups present on phytoconstituents (molecules) can be responsible for the capping and stabilizing of Ag NPs. The elemental composition confirmed the complete reduction of Ag NPs. Besides, the result demonstrated that the average particle size and zeta potential of Ag NPs were found to be 54 nm (range: 48 to 124 nm) and -50.7 mV, respectively, that confirms the uniformity and stability of Ag NPs formulation. The FTIR study demonstrated that the andrographolide is responsible for capping and efficient stabilization of NPs. Besides this, Ag NPs exhibited higher antifungal activity against Aspergillus Niger and Penicillium sp. as compared to fluconazole. In this way, it can be used as an effective antifungal agent to the control of fungal infection and there is a need to start the experiments on animal before using as an antimicrobial agent [56]. Yet another study accomplished the synthesis of spherical shape Ag NPs using fruit extract of Ficus benghalensis. The localized surface plasmon resonance of the formed Ag NPs demonstrates a peak at 402 nm in the UV-Vis spectrum that confirms the reduction of Ag+ to Ag0. Whereas, FTIR showed that the molecules containing functional groups present in the fruit extract are responsible for the reduction and stabilization of Ag NPs. The particle size (70-90 nm) and zeta potential (-33.8 mV) confirm the excellent polydispersity and stability. Additionally, the antimicrobial activity of Ag NPs paves the pathway for the suitability of these Ag NPs for biomedical applications [57]. Photochemical are the popular reducing and capping agents for Ag NPs synthesis. Kotakadi and colleagues developed the spherical, crystalline Ag NPs using aqueous leaf extract of Coleus aromaticus. Owing to the high negative zeta potential -65.7 mV, this confirms the stability of NPs formulation. Besides this, it exhibited admirable bactericidal activity against E. coli strains. They have claimed the possible mechanism for bactericidal activity. Interestingly, after the addition of NPs into a bacterial culture, the Ag NPs enter the bacterial cell by the diffusion process and by endocytosis through the cell wall into the cytoplasm. Owing to this, the NPs exhibited the toxicity to the bacterial cell constituents. Because of this, the mitochondria of a bacterial cell are damages and consequently, the depletion of ATP takes place and it promotes the production of reactive oxygen species (ROS). We witnessed that, the synergistic effect of ROS and NPs efficiently acts on the nucleus, resulted in the DNA oxidation, and finally cause the death of bacteria. Therefore, it opens the new door for the novel antibiotics against multidrug resistant strains through ecofriendly, non-toxic green synthesis [58]. Glycyrrhiza glabra (liquorice) is used in folk medicine, which contains flavanones, isoflavones, glycyrrhizo-flavonone, licoisoflavanone, glycyrin, chalcones, etc. In 2016, Kotakadi et al. synthesized the spherical and stable Ag NPs using the root extract of Glycyrrhiza glabra. In vitro assay for cytotoxic activity of Ag NPs shows the 107 % proliferation rate and 85 % of cell viability. The scanning electron microscopy images CD34+ve with Ag NPs showed excellent cell differentiation and multiplications rate. Besides this, it does not exhibit any cytotoxicity on stem cells. In the future, it can be an excellent alternative for various clinical therapy such as wound healing and in vitro growing of organs and tissues for various chronic diseases [59]. Gaddam et al. have developed uniformly spherical crystalline Ag NPs using an aqueous leaf extract of Cassia alata. The particle size and zeta potential of Ag NPs were found to 41 nm and -50.7 mV, respectively. Furthermore, synthesized Ag NPs showed minimum to moderate and good to excellent antimicrobial activity against S. aureus than E. coli. Whereas, it has been revealed exceptional antifungal activity against Aspergillus Niger and penicillium sp. In this way, the Ag NPs can be simple, cost-effective and suitable formulation for the antifungal and antibacterial application[60]. Another study reported that the synthesis of spherical shape, poly-dispersed, crystalline Ag NPs using aqueous callus extract of Gymnema sylvestre. It has been claimed that the different functional groups of phytochemicals possibly involved in the synthesis and stabilization of Ag NPs. Also, it exhibited the admirable stability due to its high negative zeta potential (-36.1 mV), which resulted into repulsion among NPs and consequently avoids the particle aggregation in formulation. The synthesized Ag NPs has been demonstrated the effective antifungal activity against Candida albicans, Candida tropicalis and Candida nonalbicans. Moreover, the Ag NPs concentration showed the proportional relationship for antifungal activity. In that, the Ag NPs perturb the cell wall that result in the release of cell wall components such as intracellular proteins and ions and finally, the growth of fungi is inhibited. At conclusion, it explores the new platform for Ag NPs and can be use as a as tropical antifungal agents in formulation of several antifungal skin ointments [61]. Yet another study has been reported the green synthesis of Ag NPs using aqueous leaf extract of Indigofera hisruta L. it showed the average particle size of 5–10nm with, good stability, and spherical shape. Also, it demonstrated the dose dependent cytotoxicity against various cancer such as prostate cancer (PC3) (IC50¼68. 5 lg/mL), mouse melanoma (B16F10) (IC50¼80.9 lg/mL). Beside this, it exhibited the antioxidant and antimicrobial potential. Therefore, in future it can be effectively used as three in one system for several biomedical applications [62]. Yet another work was developed the stable round shape Ag NPs from the aqueous fruit extract of Terminalia belarica and has been studied for its triple in one system. The FTIR spectra revealed that hydroxyl, phenols, a stretch of aldehydes, alkenes, and aromatics offers the reducing potential and converted into stable Ag NPs. Additionally, it exhibited the (3 in 1 system) antioxidant activity, antimicrobial activity, anti-proliferative, cytotoxic effect (IC50 value of 73.18 μg/mL). Therefore, the biosynthesized Ag NPs could be used as a source for the anticancer, antibacterial and antioxidant agent [63]. In this study, Palle et al. studied that the significant results were observed when Ag NPs were synthesized using leaf extract of Boerhavia Erecta. The Ag NPs are in an average of 15.9 nm, indicating its polydispersed nature. On the other hand, the negative zeta potential contributes to the Ag NPs formulation stability. The FTIR study confirmed that the synthesized Ag NPs were surrounded by proteins and metabolites viz., terpenoids having functional groups, confirmed that the carbonyl groups from the proteins and amino acid residues have the stronger ability to bind silver metal indicating that the proteins could form the Ag NPs to prevent NPs agglomeration and thereby stabilize the medium. The disc diffusion assay of Ag NPs has been confirmed the antimicrobial potential and gram-positive and gram-negative microorganisms. In addition to this, it showed the cytotoxicity to the ovarian cancer PA-1 cell line at the concentration of 25 μg/mL. Hence, based on the evidence of study, the biosynthesized Ag NPs can be good alternative to the anticancer and antimicrobial agents [64]. Similarly, in a recent attempt Panja et al. synthesied the spherical shape, crystalline Ag NPs using leaf extract of Ehretia laevis Roxb, which was in the range of 25 to 35 nm diameter. Interestingly, it showed the larvicidal activity (70±10.24%) against Culex quinquefasciatus larvae after 72 h treatment at concentration of 25 μg/mL. Beside this, it has been exhibited the anticancer activity against HeLa, human cervical cancer cells, and MCF-7 human breast cancer cells and resulted into the about 12.7 μg/mL and 14.5 μg/mL median lethal concentration of Ag NPs, respectively. Additionally, it divulged the dye degradation activity, which showed ~ 85% Congo red degradation within 8 h at a concentration of 200 μg/mL. Hence, it can be use an effectively in the biomedical as well as industrial application [22]. In a pioneering work, Jeyasree et al. developed the Ag NPs using leaf extract of Hibiscus sabdariffa. It exhibits the dose dependent bioaccumulation in tissues of Labeo rohita. The considerable alterations in the Ag NPs treated fish was confirmed the hematological activity. Beside this, the damages in the tissues was confirmed the toxicity of Ag NPs. Therefore, Ag NPs can be toxic to the ecosystem and ultimately to the human. Hence, there is huge need to optimize the dose of Ag NPs, which can exhibits the good pharmacological actions [65]. In 2018, Palithya et al. syntheised the ace-centered cubic, spherical shape Ag NPs using leaf extract of Andrographis serpyllifolia. The average particle size and zeta potential of biosynthesized Ag NPs was found to be 6 nm and -27.2 mV, respectively. They claimed that the N-H stretching of amides, carboxylic acids, ether groups, O-H hydroxyl groups, and C−O stretching of alcohols reduced and stabilize the Ag NPs. In addition, it exhibited the antibacterial and antioxidant activity. Therefore, it concluded that the Ag NPs can be potential alternative for the several biomedical applications [66]. The authors have investigated the in-vitro Cytotoxic and Genotoxic Properties of biosynthesized Ag NPs. In this, the spherical shape Ag NPs have been synthesized using latex extract of Allamanda cathartica. The high negative (–27.6 mV) zeta potential was shown by Ag NPs, which confirms the stability of Ag NPs. In addition, the strong absorption peak in the visible range (455nm) was found due to surface plasmon vibrations excited. We witnessed that the size and shape of Ag NPs reflect the absorbance peak. In fact, the size has a linear correlation with the UV peak intensity, while the number of Ag NPs does not have such linear correlation. The FTIR demonstrated that the water-soluble essentials secondary metabolites are responsible for capping and efficient stabilization of Ag NPs. The cytotoxicity of Ag NPs confirmed the concentration-dependent toxicity, in that the Ag NPs may disrupted normal cellular functions, that influencing the membrane integrity and finally inducing programmed cell death. The DNA fragmentation assay showed that the Ag NPs exhibited the poptotic fragmentation of lymphocyte DNA, which may due to interaction between functional groups of proteins and DNA and silver atoms. Overall, the green Ag NPs showed very good cytotoxic and genotoxic activity in peripheral blood mononuclear cells. In future, it could be novel alterntive to the treatment of peripheral blood mononuclear cells and deoxyribonucleic acid fragmentation in children [67]. From its inception, Couroupita guianensis Aubl. is utilized for the treatment of various diseases due to their noteworthy properties viz., anti-inflammatory, anti-oxidative, anti-cardiac, antidiabetic, antibacterial and anticancer activities. In 2018, Akther et al. reported the rapid and cost effective synthesis of spherical shape monodispersed Ag NPs using anther extract of Couroupita guianensis (C. guianensis) Aubl. The phenolic compounds possess the ability to reduce Ag+ to Ag0 and it may due to its electron donating property. The FTIR of Ag NPs divulged that nitrile and carboxyl groups has been efficiently bind with heavy metals and due to the presence of these functional groups it suggested that Ag NPs were stable in formulation. Further, they achieved the good antibacterial activity against gram positive and gram-negative bacterial pathogens that showed the significant growth inhibition as well as biofilm formation. Concisely, anthers of C. guianensis are a potent biosource for biosyntheised Ag NPs and it paves the pathway for potent alternative to antibiotics [68]. Based on the noteworthy finding of research it concludes that the green synthesis of Ag NPs is an easy, simple, cost-effective, and eco-friendly path for NPs fabrication as compared to the previously reported methods. Consequently, the plant extract played a massive and key role in the Ag NPs green synthesis. Along with that, the several attributes for Ag NPs synthesis were enlisted in table 1, that could enhance the quality and quantity of Ag NPs.
Table 1 Plant Extract as a reducing agent
|
Sr. No. |
AgNO3 concentration |
Green source |
Extract (mL) |
AgNO3 (mL) |
Particle size (nm) |
Ref. |
|
|
1 mM |
Leaf extracts of Moringa oleifera |
5 |
50 |
9-11 |
[30] |
|
|
2mM |
Ginger rhizome, Thyme’s leaves |
1 |
20 |
12-18 |
[31] |
|
|
0.5mM |
O. vulgare L. aerial parts extract |
1 |
49 |
2-25 |
[32] |
|
|
1mM |
Oryza sativa husk extract |
5 |
90 |
-- |
[33] |
|
|
5Mm |
Argemone maxicana leaf extract |
10 |
90 |
30 |
[34] |
|
|
10mM |
Seed extract of Alpinia katsumadai |
15 |
150 |
12.6 |
[35] |
|
|
0.1M |
Leaf extract of Clitoria ternatea and Solanum nigrum |
5 |
45 |
20 &28 |
[36] |
|
|
1mM |
S. apetala Leaves Extract |
20 |
80 |
27.3 |
[37] |
|
|
1-4mM |
Hibiscus leaf extract |
10 |
190 |
30-40 |
[38] |
|
|
0.1mM |
Flower extract of Roheda |
5 |
500 |
5-78 |
[39] |
|
|
1mM |
C. halicacabum L. leaves extract |
1 |
9 |
100 |
[40] |
|
|
1mM |
Raspberry leaf extract |
10 |
- |
100 |
[41] |
|
|
1-3mM |
Seed extract of C. esculentus and B. paradoxum |
- |
- |
-- |
[42] |
|
|
1mM |
G. glabra Root Extract |
2 |
98 |
19 |
[43] |
|
|
3mM |
Aqueous extract of P. anisum seeds |
2.5 |
250 |
3.2-16 |
[44] |
|
|
1mM |
Leaf Extract of Rosemary |
50 |
- |
17 |
[45] |
|
|
1mM |
A. andrachne leaf water extract |
10 |
100 |
107 |
[46] |
|
|
1mM |
Fruit extract of P. emblica And C.grandis |
5 |
95 |
-- |
[47] |
|
|
7mM |
Fruit extract of sapota pomace |
1 |
05 |
8-16 |
[48] |
|
|
5mM |
Extract of leaves of Neem, Tea, and Peels (kitchen waste) |
1 |
5 |
-- |
[49] |
|
|
0.1M |
Leaf extract of mint |
15 |
10 |
26 |
[50] |
|
|
1mM |
Satin leaf extract |
1 |
100 |
25 |
[51] |
|
|
1mM |
Fresh leaves of Volkameria inermi |
10 |
90 |
50 |
[52] |
|
|
1mM |
Eriobotrya japonica (Thunb.) leaf |
1 |
1,2,10 |
9.2 |
[53] |
|
|
5mM |
Crude latex by stems of J. curcas plant |
20 |
20 |
20-30 |
[54] |
|
|
0.025 M |
Dried leaves of Catharanthus roseus |
5 |
10 |
27-32 |
[55] |
|
|
0.025 M |
Dried leaves of Andrographis paniculata |
10 |
20 |
54 |
[56] |
|
|
1 mM |
Fruit extract of Ficus benghalensis |
-- |
-- |
70- 90 |
[57] |
|
|
0.001 M |
Aqueous leaf extract of Coleus aromaticus |
5 |
10 |
48 |
[58] |
|
|
0.025M |
Root extract of Glycyrrhiza glabra |
1 |
10 |
41.5-46.5 |
[59] |
|
|
0.025 M |
Aqueous leaf extract of Cassia alata |
5 |
10 |
>41 |
[60] |
|
|
1 mM |
Aqueous callus extract of Gymnema sylvestre |
10 |
90 |
3-30 |
[61] |
|
|
0.025 mM |
Aqueous fruit extract of Terminalia belarica |
2 |
20 |
46.5 |
[63] |
|
|
0.01mM |
Leaf extract of Boerhavia Erecta |
1 |
10 |
15.9 |
[64] |
|
|
1 mM |
Leaf extract of Ehretia laevis Roxb. |
1 |
9 |
25-35 |
[22] |
|
|
- |
leaf extract of Hibiscus sabdariffa |
10 |
200 |
-- |
[65] |
|
|
0.002M |
Leaf extract of Andrographis serpyllifolia. |
- |
10 |
20-100 |
[66] |
|
|
1 mM |
Aqueous atex extract of Allamanda cathartica |
0.6 |
20 |
35 |
[67] |
|
|
1 mM |
Anther extract of Couroupita guianensis Aubl. |
1 |
1 |
10-45 |
[68] |
Microorganisms as a Reducing Agent
Fungus
The arena of nanotechnology has witnessed impressive advances in various biomedical applications. Owing to its noteworthy outcomes, from the past few decades, the investigator is focused on the field of nanotechnology. Due to the advantage of green synthesis, investigators introduce the new area for green synthesis of Ag NPs from various microorganisms including fungus, algae, etc. The present investigation was claimed that victorious achievement due to the successful synthesis of Ag NPs by fungus. In brief, the authors were conquering the crisis of chemical methods by bioinspired Ag NPs synthesis using the fungus, namely, Verticillium (isolated from the Taxus). The possible reduction mechanisms of silver ions were confirmed by using electron microscopy analysis. It confirms the formation of Ag NPs below the cell wall surface. Enzymes present in the cell wall membrane of the fungus that engaged the synthesis process of Ag NPs. The finding of the investigation reported that the NPs were nontoxic, spherical in shape as well as nanometer in size. Thus, the present scientific report enhanced the researcher attraction in microorganism applications in the arena of green synthesis of Ag NPs [69]. Ahmad and co-authors were demonstrated the bioinspired synthesis of Ag NPs using fungus species Fusarium oxysporum (F. oxysporum). The fungus provides the potential for the reduction of silver. It showed the nanosized specification and the excellent morphology of NPs. Also, the authors claimed that the release of fungus containing reductase enzyme which is responsible for the reduction of silver ions and fabricates the Ag NPs. In addition, the reduction of silver ions does not affect the fungus. Then these prepared NPs showed the long-term stability for Ag NPs and the probable reason behind this is a stabilization of the Ag particles by the proteins or the possibility of the strong interaction of Ag NPs and enzymes (for example cytochrome c.) [70]. Nature is having a huge sum of versatile microorganism, which provides the oxidation and reduction potential for the various toxic metals. Hence taking to the consideration of these advantages authors were fabricated Ag NPs using the Aspergillus fumigates (A. fumigatus). The exact mechanism is still mysterious but the established probable mechanism shown the authentication for fungus containing NADH dependent reductase was involved in the reduction of silver ions. Additionally, the interaction of NPs with proteins enhances the stability of Ag NPs [71]. Yet another work revealed that the use of Phaenerochaete chrysosporium (P. chrysosporium) for the reduction of Ag NPs. Briefly, the bioinspired synthesis of Ag NPs mechanism is based on biosorption and reduction of metal ions. Authors were reported the various possible general mechanisms involved in the synthesis of NPs include the adsorption of metal ions on the microbial surface by chemical functional groups on the cell wall biopolymers (example: carbonyl group). Also, in situ reduction of reduced to silver ions due to the presence of reducing sugars from hydrolysates of polysaccharides, enzymes. It has been claimed that the protein and enzymes provide the ability to reduce, stabilizing, and capping capacity towards the Ag NPs[72]. A similar line of work was carried out using the Aspergillus flavus (A. flavus) as reducing media. In brief, authors were reported that the microbes containing amino acids specially present in proteins/enzymes, such as aspartic acid and tryptophan showed the reduction potential to the metal ions [73]. Gajbhiye M. and co-authors were investigated that the use of Alternaria alternate (A. alternate) in the synthesis of Ag NPs. Briefly, the presence of free amine groups, electrostatic attraction, cysteine residues, and mycelial cell wall enzyme-containing negative charge carboxylate groups provides the NPs reduction capping and stabilization. It showed admirable antifungal activity against Phoma glomerata, Phoma herbarum, Fusarium semitectum, Trichoderma sp., and Candida albicans[74]. The Cryphonectria species of fungus has been used for the fabrication of Ag NPs which showed the excellent potential for the reduction of silver ions. The possible mechanism of green synthesis of NPs has been similar to the previously reported one. As a result, these synthesized NPs give the highest antimicrobial activity against both S. aureus and E. coli as compared to S. typhi and C. albicans [75]. Yet one more report has been revealed the use of Aspergillus Niger for the biosynthesis of Ag NPs. The FTIR study of prepared NPs showed the characteristic peak of protein and amino acids which confirmed that the extracellular proteins secreted by the fungus are responsible for the reduction, stabilization, and capping of silver ions. Also, the reductase enzyme of fungus showed a key role in the Ag NPs synthesis which was confirmed by nitrate reductase assay. Finally, the prepared NPs were checked for the antibacterial and antifungal activity. It showed enormous potential against the strain of fungi and bacteria [76]. Non-hazardous, competent, and green synthesis of Ag NPs has been carried out using the fungi species Amylomyces rouxii strain, KSU-09 which isolated from the roots of date palm (Phoenix dactylifera). It demonstrated the antimicrobial potential against various pathogens by oligodynamic effect via denaturing the cellular proteins, inhibition of DNA replication. Also, NPs could alter the cell membrane permeability of pathogens [77]. Shahzad and co-authors revealed the fungus A. fumigates species based bioinspired synthesis of Ag NPs. authors reported the various optimization parameter effect on the NPs synthesis and its properties. Briefly, optimization parameters including temperature alter the reaction rate of NPs, based on observation, it concludes the increasing the temperate alter the particle size. The high particle size of NPs enhanced the aggregation of NPs. The fresh and new culture provides superior result as compared to the old culture. It reduced the reaction time of synthesis. Thus, the culture age is a major parameter that affects the size and shape of NPs. Besides, the optimum pH 6 for the fabrication of Ag NPs reported by authors. Also, microbes contain NADH (Nicotinamide Adenine Dinucleotide Hydrogen) having a pH 6.5-7 and because of this, the enzymes play an essential key role in the synthesis of Ag NPs. The high concentration of substrate leads to enlarging the size and aggregation of Ag NPs and the high biomass reduced the size of NPs and reaction time. Besides this, the high concentration of metal salt reduced the particle size and reaction time. Finally, fabricated Ag NPs showed the least reaction time possessing antibacterial effect against medically important resistant bacterial strains. Also, it demonstrated the slight antiproliferative effects on the cancer cell line [78]. For the last decades, the investigator has majorly focused on the possible interaction between inorganic metal and biological samples. A number of the report found on the reduction of metal by microorganisms and based on these hypothesis authors was fabricated probiotic i.e. Bacillus licheniformis (B. licheniformis) cell-free extract (BLCFE) coated Ag NPs. In brief, The NADH and NADH-dependent nitrate reductase enzyme showed the ability to reduce the silver ions. In conclusion, it showed efficient activity against V. parahaemolyticus Dav1 [79].
Table 2 Fungus as a reducing agent
|
AgNO3 concentration |
Green source |
Extract volume (mL or g) |
AgNO3 volume (mL) |
Particle size (nm) |
Ref. |
|
4×10-4 M |
Verticillium |
100 |
100 |
25 |
[69] |
|
1 mM |
F. oxysporum |
100 |
100 |
5-50 |
[70] |
|
1 mM |
A. fumigates |
50 |
-- |
5-25 |
[71] |
|
1 mM |
P. chrysosporium |
-- |
-- |
50-200 |
[72] |
|
1 mM |
A. flavus |
5g |
100 |
8.92 |
[73] |
|
1 mM |
A. alternata |
100 |
-- |
32.5 |
[74] |
|
1 mM |
genus Cryphonectria |
100 |
-- |
30-70 |
[75] |
|
1 mM |
Aspergillus niger |
100 |
-- |
3-30 |
[76] |
|
1 mM |
Amylomyces rouxii |
10g |
100 |
5-27 |
[77] |
|
1 mM |
A. fumigatus |
1 |
1 |
0.68 |
[78] |
|
1 mM |
B. licheniformis |
-- |
-- |
18-63 |
[79] |
Bacteria
Based on the advantages of green synthesis in nanotechnology, it releases a new arena for nano biomedical applications. Form the last few years, enhancing the utilization of microorganisms in nanotechnology for the reduction of metal ions. Herein, the victorious attempt has been reported by authors to made Ag NPs using Escherichia coli, (E. coli top 10) as a reducing agent. These prepared NPs showed a toxic effect against bacteria. Furthermore, it has been claimed the probable mechanism includes alterations in the cell membranes and interaction of the Ag NPs with cell components (DNA and protein). Thus, it proposes a new proposal to enlarge the research area for antimicrobial agents against non-metabolically active bacteria species [80]. Kulkarni R. and coauthors were reported that the use of Deinococcus Radiodurans (D. Radiodurans) in the synthesis of Ag NPs. Owing to the presence of amide linkage and other functional groups explored the fabrication of Ag NPs. Also, the authors claimed that the proteins/ peptides of D. radiodurans give stability to Ag NPs. These prepared NPs showed a high potential against gram-negative bacteria than the gram-positive bacteria [81]. Yet another work was reported that the use of Gluconacetobacter xylinus (G. xylinus) in green synthesis of Ag NPs. In brief, immersing bacterial cellulose in the AgNO3 solution, it gives the impregnated Ag NPs in bacterial cellulose. Due to the presence of a variety of reducing agents in cellulose, the in situ reduction of Ag+ occurred and Ag NPs were fabricated. In addition, it has been reported that the OH- the addition has a significant role to metallic ions for its reduction reaction. Besides, it is probable to amplify the reducing power of compound under alkaline circumstances than the standard reducing potential of the same compound [82]. The psychrophilic bacteria including Pseudomonas Antarctica (P. Antarctica), Pseudomonas proteolytica (P. proteolytica), Pseudomonas meridian (P. meridian), Arthrobacter kerguelensis (A. kerguelensis) and Arthrobacter gangotriensis (A. gangotriensis) were fruitfully used in the stabilization of Ag NPs. Briefly, the use of nutrient broth to convert AgNO3 to Ag NPs and after that, the Ag NPs was stabilized by cell-free culture supernatants of bacteria. Accordingly, authors were claimed that this type of extracellular development of Ag NPs could be more beneficial to the commerce [83]. Another report explored the application of the extracellular microbe mediated synthesis of Ag NPs by Serratia nematodiphila (S. nematodiphila). This prepared Ag NPs showed excellent antibacterial activity against B. subtilis, Klebsiella planticola, and P. aeruginosa [84]. Bioinspired, simple, cost-effective green synthesis of Ag NPs was carried out by using culture supernatant of Nocardiopsis sp. MBRC-1. These prepared NPs showed the antibacterial and antifungal potential for pathogens [85]. The numerous investigations were reported that the use of bacteria and fungi masses in the fabrication of Ag NPs. But biosynthesis is a more time-consuming process. Authors were claimed and proved that the utilization of K. pneumonia, E. coli, and Enterobacter cloacae (Enterobacteriaceae) in Ag NPs synthesis. It required less time for synthesis as compared to the previously reported method. Due to the presence of the nitro-reductase enzyme, the reaction rate accelerated and the silver ions reduced [86]. Due to versatile and unique properties, the novel combinatorial synthesis approach has been used in the synthesis of NPs. Saifuddin and co-authors were reported that green synthesis of Ag NPs using B. subtilis and microwave irritation with combinatorial approach. The using B. subtilis reduced the silver ions and microwave irradiation enhanced the reaction rate of reduction of silver ions [87]. Lately, the novel enzymatic strategy has been developed via using bacteria and fungi in the fabrication of NPs, especially intra- and extracellularly. A successful attempt has been made by authors to prepared the Ag NPs by using S. aureus. These synthesized Ag NPs showed sensitive antimicrobial activity against methicillin-resistant S. aureus [88].
Table 3 Microorganisms as a reducing agent for Ag NPs
|
AgNO3 concerntration |
Green source |
Extract concerntration |
AgNO3 volume |
Particle size (nm) |
Ref. |
|
1 mM, 5 mM |
E. coli top 10 |
100 mL |
-- |
15 - 97 |
[80] |
|
1, 2.5, 5, 10 mM |
D. Radiodurans |
-- |
-- |
16.82 |
[81] |
|
0.06 M |
G. xylinus |
|
-- |
40 - 100 |
[82] |
|
1 mM |
P. Antarctica, P. Proteolytica, |
25 mg |
1 mL |
13.35 |
[83] |
|
1 mM |
S. nematodiphila |
100 mL |
-- |
10 - 31 |
[84] |
|
1 mM |
Nocardiopsis sp. MBRC-1 |
-- |
-- |
45 |
[85] |
|
1 mM |
Enterobacteriacae group |
1 mL |
-- |
52.5 |
[86] |
|
1 mM |
B. subtilis |
50 mL |
50 mL |
5 - 50 |
[87] |
|
1 mM |
Staphylococcus aureus. |
1 mL |
-- |
160 - 180 |
[88] |
Miscellaneous
Gunasundari and workers prepared the MNPs including silver, chromium, iron, lead, and zinc by green synthesis method using the Spirulina platensis (S. platensis) a species of algae as a reducing agent for metal ions. Finally, these prepared NPs were subjected to antibacterial activity. It showed excellent antimicrobial activity against gram-positive bacteria (S. aureus), gram-negative bacteria (K. pneumonia, Proteus vulgaris, P. aeruginosa, and E. coli), and fungus (A. niger) [89].
Conclusions
Since the previous decades, many efforts have been made for the eco-friendly synthesis of Ag NPs. It overcomes the limitations of chemical and physical methods of Ag NPs synthesis. It is eco-accommodating and cost-effective, and it can be scaled up for large-scale production. There has been a growing attentiveness towards the green chemistry and ecofriendly green method for synthesis of Ag NPs. Agro-waste as well as plant materials, bacteria, fungi, and algae, etc. revealed excellent potential for the synthesis of Ag NPs. Mainly, microorganisms including bacteria to complex eukaryotes can be used for fabrications of Ag NPs with acceptable particle sizes and shapes. Owing to the residual of microorganisms in nanoparticle, the research tract changed towards the utilization of plant extracts. It provides a fast production rate, lessened wastages, and more stable products, etc. Hence, the eco-friendly synthesis of Ag NPs has a key phase of nanotechnology by a variety of applications. In the future, it can be utilized for various applications including medicine, biosensors, therapeutics, implants, etc.
Acknowledgments
The authors are thankful to the Indian Council of Medical Research (ICMR), India for research funding No.5/4–5/159/Neuro/2015-NCD-I) and H. R. Patel Institute of Pharmaceutical Education and Research, Shirpur.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Sopan Namdev Nangare, Pravin Onkar Patil,. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.