TNF-α Loaded on Gold Nanoparticles as a Good Therapeutic Agent against Breast Cancer AMJ13 Cells
Noor Abood 1, Majid Jabir 2*, Haider Kadhim 3
1 Medical Laboratory Techniques, Al-Mamoon University College Baghdad, Iraq.
2 Division of Biotechnology, Department of Applied Science, University of Technology, Baghdad, Iraq.
3 Department of Micrbiology, University of Al-nahrain, Baghdad, Iraq.
* Corresponding author. E-mail: msj_iraq@yahoo.com
Received: Mar. 14, 2020; Accepted: Jul. 3, 2020; Published: Sep. 10, 2020
Citation: Noor Abood, Majid Jabir, and Haider Kadhim, TNF-α Loaded on Gold Nanoparticles as a Good Therapeutic Agent against Breast Cancer AMJ13 Cells. Nano Biomed. Eng., 2020, 12(3): 262-271.
DOI: 10.5101/nbe.v12i3.p262-271.
Abstract
Although the remarkable anti tumor effect of tumor necrosis factor (TNF-α) and the essential role in diverse cellular and immunological properties have been evidenced, the clinical use of TNF-α is hindered due to its toxicity. Our study was aimed to develop a new drug delivery system by binding pygelated gold nanoparticles (50 nm) with TNF-α and then investigate the anticancer activity against AMJ13 cell line. The binding of these compounds were confirmed and characterized using ultraviolet-visible spectroscopy (UV-Vis), scanning electrone microscope (SEM), and transmision electrone microscope (TEM). Varios parameters in vitro were used to examine the anticancer activity of each compound against AMJ13 cell line. Gold nanoparticles (GNPs) and TNFα-GNPs were found to exert cell growth arrest against the cancer cell line. The anti-proliferative effect of these compounds was due to cell death and inducing apoptosis as confirmed by using 4',6-Diamidino-2-phenylindole (DAPI) fluorescent assay, flow cytometry assay, and finally mitochondrial membrane potential (MMP) staining, Real-time polymerase chanin reaction (RT-PCR) was used to detect changes in the expression of p53 protein. In addition, we studied the effect of drug delivery system on body weight on mice. In conclusion, the results of this study demonstrated that the TNFα-GNPs inhibited AMJ13 cells proliferation, resulting in apoptosis during novel pathway that involved mitochondrial damage and up-reglulated p53. Taken together, the results suggested that the TNFα loaded GNPs could be a promising therapy protocol for cancer cells and could be used for wide medical applications and offer new drug recompensing a chemotherapy drug.
Keywords: GNPs; TNF-α; TNF loaded GNPs; AMJ13 cells; Cytotoxicity; MMP; p53 expresion
Introduction
Cancer is the second leading cause of death, following heart diseases [1]. The global cancer burden is estimated to have risen to 18.1 million new cases and 9.6 million deaths in 2018 [2]. The rising incidence of cancer depends on several risk factors such as increased pollution [3], radiation, lack of exercise and a balanced diet, and genetics [4]. Any of these factors can lead to a mutation in the DNA of cells and develop into cancer. Most common types of treatment against cancer include chemotherapy, surgery, radiation, and a combination of any of these treatments [5]. However, non-specificity and toxicity are challenges associated with these traditional treatments [6]. The application of nanotechnology in cancer treatment has the potential to solve these limitations. Designing nanoparticles loaded with multifunctional drugs, and functionalizing their surfaces with recognition proteins can target specific cancer cells [7, 8]. The advantages of such targeting include the drug amount needed to achieve a therapeutic effect may be significantly reduced as well as the drug concentration on the cancer site can be increased without any bad effects on healthy cells [9]. Nanoparticles based on gold chemistry have attracted significant research and practical consideration lately. GNPs flexible agent with a selection of many biomedical applications including use in highly sensitive analytical assessments, ablation thermal and radiotherapy development, as well as drug and gene delivery. For biomedical uses, external functionalization of gold nanoparticles (GNPs) is necessary in order to target them to specific disease areas and allow them to selectively interact with cells or biomolecules. The resulting GNPs have unique properties, such as morphology, size, electronic characteristics, a high surface area to amount ratio, and surfaces that can be readily modified with ligands containing functional groups such as thiols, phosphines, and amines, which display affinity for gold surfaces [10]. Tumor necrosis factor-α (TNF) was initially characterized as the active component in endotoxin-induced hemorrhagic necrosis of transplanted tumors [11]. TNF has since been described as a pleiotropic cytokine with diverse cellular and immunological properties and associated pathologies [12]. Administration of recombinant human TNF (rhTNF) has been investigated for the treatment of solid tumors due to vascular damaging and immune-stimulating properties of the protein. As an anti-tumor drug, TNF has been limited clinically due to systemic toxicity [13, 14], giving rise to the need for a selective tumor delivery mechanism. On the other hand, polyethylene glycol (PEG) is nowadays one of the most used biopolymers, being a principal component in different classes of therapeutic agents that are already in clinical use. It is inexpensive, versatile, and FDA approved for clinical use in the USA [15]. PEG-coated gold nanoparticles to enhance the avoidance of these particles to be uptake and cleared by Reticuloendothelial system (RES) [16]. In the present study, we designed a drug delivery system consisting of gold nanoparticles that were coated with PEG, combined with TNF-α, and then we investigated their anti-proleferative activity against Iraqi breast cancer cell line AMJ13 with a study the possible mechanisms of this effect which involved p53 pathway.
Experimental
Chemicals and reagents
The human breast AMJ13 cell lines were obtained from ALMustansiriyah University, Baghdad [17], Iraq. Acridine orange, ethidium bromide, trypsin-EDTA, fetal bovine serum, DMSO, MTT, and crystal violate stain were obtained from Sigma (St. Louis, MO, USA). RPMI-1640 medium was purchased from Gibco (USA). Tumor necrosis factor-α Protein (human recombinant) was purchased from Sigma Aldrich (Germany). Gold nanoparticles (30 nm diameter, methyl terminated) coated with polyethylene glycol 5000 purchased from Sigma Aldrich (Germany). PBS was obtained from OXOID (England). MitoCaptureTM Apoptosis Detection Kit was purchased from CalBiochem (San Diego, CA), Penicillin and streptomycin from Biosource International, Belgium. All the other chemicals and reagents were used at the analytical grade level.
Loading of TNF-α on PEGylated gold nanoparticles
An aqueous solution of TNF-α was reconstituted in normal saline (0.45% NaCl) to a concentration of 80 µg/µL (W/V stock solution) and 10 ng/mL of TNF-α mixed with gold nanoparticles solution (10 µg/mL). The mixture was then inocubated for 24 h. The pH of the mixture was adjusted to be 8, which allowed TNF-α to bind to the colloidal gold particles [18]. The mixture was incubated at room temperature for 24 h.
Investigation of the bindig between TNF-α on PEGylated gold nanoparticles
ELISA test was done to detect the binding between GNPs and TNF. In brief, GNPs at concentration 10 µg/mL were mixed with TNF-α at concentration 10 ng/mL at the ratio of 15 : 1. The mixture was incubated overnight and then centriguged 10000 prm for 20 min. ELISA assay was done to measure TNF concentration in supernatant and pellet. Briefly, 100 μL from standard and sample were added to each well. Then, the microplate was incubated for 90 min at 37 °C, followed by removing all extra liquid. Thereafter, a 100 μL of biotinylated detection antibodies were added, followed by incubation period for 1 h at 37 °C. Then, the micro ELISA plate well was aspirated and washed 3 times. After the washing step, 100 μL HRP conjugate was added and incubated for 30 min at 37 °C. There were another aspiration and washing step for 5 times, followed by adding 90 μL substrate reagent and incubating for 15 min at 37 °C. And finally, 50 μL of stop solution was added. Read at 450 nm by using ELISA microplate reader (Organon tekinka, Austria), the results were collected [19], data not shown.
Characterization of the binding of GNP with TNF-α
Scanning electron microscopic and transmission electron microscopy
SEM and TEM were used to visualize the morphology and nanoparticle grain size of gold nanoparticles. Thin films of gold nanoparticles were prepared on a cover slide grid by dropping the amount of solution on the cover slide, then allowed to dry at room temperature before being visualized under SEM [20].
Ultraviolet-visible (UV-Vis) spectrophotometer analysis
The UV-Vis analysis was determined by scanning the prepared samples using the UV-Vis spectrophotometer (Metertech SP-8001, Taiwan), which ranged from 200 - 1100 nm [21].
Anticancer activity of TNF-α loaded on GNPs
Cytotoxicity determination using MTT assay
Cells were seeded at 1× 105 cells/mL in 96 well microtiter plates in RPMI medium [22]. The cells were incubated overnight for attachment. GNPs and TNF-α loaded on GNPs were added to wells and incubated for 24, 48 and 72 h. Thereafter, the cells were treated with MTT. After the incubation time, all the contents of the well were aspirated. DMSO was added to each well after the incubation period and the absorbance was measured at 490 nm using a microplate reader [23]. The growth inhibition rate was extracted as in the following equation:
% Inhibition rate = (A - B/A) × 100,
where A and B represent optical densities for the control and treated samples, respectively [24].
DAPI staining for the nucleus
For this test, cells were subjected to 12-well plating, followed by 24 h incubation with TNF-α loaded on GNPs, PBS washing, and 30 min fixation with 1% glutaraldehyde. After removal of fixative with PBS, 30 min DAPI staining was performed, followed by another PBS washing, and the final fluorescence microscopic observation of the morphology of the nucleus microscopy BX51 UV fluorescent microscope (Olympus, Tokyo, Japan) [25].
Flow cytometry assay
Flow cytometry assay was used for apoptosis detection using. AMJ13 cells treated with TNF-α loaded on GNPs were analyzed by determining the ratio of cells with nucleus concentration and fragment. Cells were seeded for 24 h then treated with TNF-α loaded on GNPs. AMJ13 cells were suspended in the FACS buffer. Cells were stained with annexin V-FITC (Invitrogen, Carlsbad, CA) and determined by flow cytometer.
Mitochondrial membrane potential assay
Simultaneous determination of the critical apoptotic events in the cells was treated with 2-benzhydrylsulfinyl-N-hydroxyacetamide. JC-1 dye was employed for probing the membrane potential of the mitochondria. In brief, 24 h seeding of cells in 96-well plates was followed by treatment with the IC50 dose of the extracted active compound and staining with MMP dyes [26].
Detection of gene alteration using quantitive real time PCR (qRT-PCR)
Gene alteration of cell line was investigated using real-time quantitative PCR. In this experiment, p53 gene was measured to identify the pathway of apoptosis and the mechanism action. RT-PCR was accomplished to investigate the modifications in hippocampal expression genes. The primer sets were designed based totally on the sequences from the NCBI database. The sequences of primers used within the quantitative RT-PCR assay included p53, forward: 5’-CCGTCCCAAGCAATGGATG-3’, reverse: 5’-GAAGATGACAGGGGCCAGGAG-3’. Each RT-PCR reaction combination contained 1 µL cDNA, 7.5 µL SYBR green, 0.3 µL ROX, and 0.3 µL related primers. The final quantity was topped up to 15 µL via adding 5.6 µL of distilled water. The assay was performed with SYBR Premix Ex. TaqTM kit. The real-time detection of emission intensity of SYBR green reacted to double-stranded DNAs and was performed via the implemented Biosystems ABI PRISM Sequence Detection System. GAPDH mRNA was used as an inner control to identify the relative expression amount of genes [27].
Toxicity of TNF-α loaded on GNPs in mice
Balb/c mice (20-30 gm, 7-9 weeks) were divided into 3 groups (n = 5), and kept in polypropylene cages with wood dust under standard experimental conditions (24 - 26 oC, 55 - 65% RH, 12 h light/dark cycle), while food and water were supplied ad libitum. The experiments were approved by the Animal Ethical Committee, Biotechnology Division, Applied Science Department, University of Technology, Baghdad, Iraq. In Group 1 (control), intraperitoneal (i.p.) injection of PBS (250 µL) was applied, whereas Groups 2, 3, received i.p. injections of GNPs and TNF loaded on GNPs respectively at concentration of 0.5 mg/kg for 4 weeks. Following the end of injection periods, bodies of the mice were measured [28].
Statistical analysis
The study data were presented as means ± standard error of the mean. The statistical analyses were performed using the GraphPad Prism 5 software package (GraphPad Software, Inc. San Diego, California) [29].
Results and Disscusion
Loading of TNF-α to GNPs
The GNP used in this experiment was coated with poly ethylene glycol that had a methoxy end group (OCH3) at the free end as shown in the Fig. 1. This group was bound with amine group (NH2) of lysin amino acid of TNF-α cytokine. The PH of solution was adjusted to 8 which was slightly basic. At this PH, the protein had a negative chagre and the layer of methoxy poly ethylene glycol (mPEG) was at pH 7 which was neutral and would become positively charged.
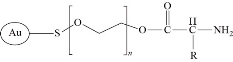
Fig. 1 The chemical structure of GNP-mPEG bound to amine group of lysin amino acid of TNF-α.
Scanning electron microscopy and transmision electron microscopy
SEM and TEM techniques were used to visualize the size and shape of the GNP and GNP loaded TNF-α. The images of SEM and TEM for both compunds are shown in Fig. 2. These images showed relatively spherical shaped nanoparticles with a diameter around 30 nm for GNP and TNF-α loaded on GNPs, respectively. SEM and TEM results showed the agglomeration of molecules after binding the TNF-α and GNPs compared to GNP molecules alone that appeared as separated grains.
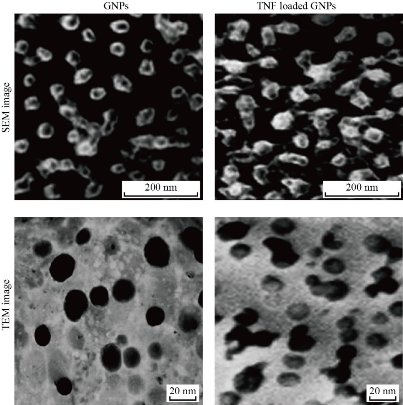
Fig. 2 SEM and TEM images of GNP and TNF-α loded GNPs.
Ultraviolet-visible spectroscopy
Ultraviolet-visible spectroscopy (UV-Vis) absorption technique refers to the measurement of the attenuation of electromagnetic radiation by an absorbing substance; it can specify the difference between compounds in the absorption that may be shifted. The solutions were observed under a UV-visible spectrophotometer and the results are shown in Fig. 3. The sharp bands of gold nanoparticles were observed almost 520 nm. TNF-α was attached to pegylated gold nanoparticles; the top band was shifted and a very clear wide band appeared.
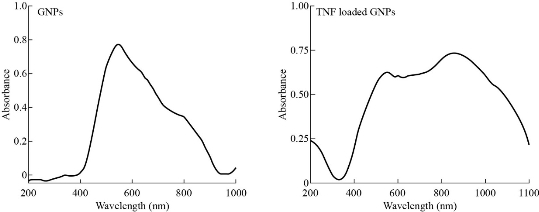
Fig. 3 UV-Vis of GNP and TNF-α loaded on GNPs.
Cytotoxicity determination using MTT assay
MTT assay is a colorimetric method based on a color change by metabolically-active cells. The cytotoxic effects of GNPs and GNP-TNF-α on the viability of human cancer cell lines (AMJ13) were studied for 48 h and presented in Fig. 4. The results revealed a significant inhibition of AMJ13 proliferation after 48 and 72 h. The cell proliferation was significantly lower compared to the untreated control cells. After 48 h of treatment with GNP-TNF-α at concentration of 10 µg/mL and 10 ng/mL of GNP and TNF-α respectively, more than 50 % of AMJ13 cell lines were killed. On the other hand, after 72 h, the cytotoxicity was mor than 75%. Low cytotoxicity showed on AMJ13 cell line when treated with GNPs at the same concentration and time. These compounds revealed no significant effect against normal REF cell line. The results revealed that these compounds had a potential effect as antiproliferative and cytotoxic substances. In addition, these compounds had an apoptotic property and it was examinated through morphological changes on the AMJ13 cell line using an inverted phase contrast microscope. As seen in Fig. 4, the control (untreated) cells maintained their original morphology and were mostly adhere to the tissue plate. Conversly, GNPs and GNP-TNF-α exhibited high antiproliferation activities and morphological changes showed on the cells after 48 h (Fig. 5). The apoptotic characteristcs such as membrane blebbing reduced the number of cells and loss of contact with adjacent cells was noticed. This suggested that GNP-TNF-α had a potential role as anti-cancer activity for in-vivo applications. Goel et al. [30] demonstrated that bioactive TNF-α from CYT-6091 was preferentially taken up by the tumor, with subsequent accumulation of Au in liver and spleen after TNF-α bioactivity dropped in blood. Their data also provided evidence of TNF-α binding to the tumor tissue. Overall, CYT-6091 exhibited significant improvements in the targeted delivery of TNF-α to the tumor tissue. The improvements included enhanced and selective uptake of TNF-α by the tumor tissue, and the absence of observable side effects from either the therapeutic drug or the carrier. GNPs, has been used as a therapeutic for the treatment of cancer as well as an indicator for immunodiagnostics. However, the use of these gold nanoparticles for in-vivo drug delivery has never been described. This communication outlined the development of a colloidal gold (cAu) nanoparticle vector that targeted the delivery of tumor necrosis factor (TNF) to a solid tumor growing in mice [19]. The optimal vector, designated PT-cAu-TNF, consisted of molecules of thiol-derivatized PEG (PT) and recombinant human TNF that were directly bound onto the surface of the gold nanoparticles. Following intravenous administration, PT-cAu-TNF rapidly accumulated in MC-38 colon carcinoma tumors and showed little to no accumulation in the livers, spleens or other healthy organs of the animals.
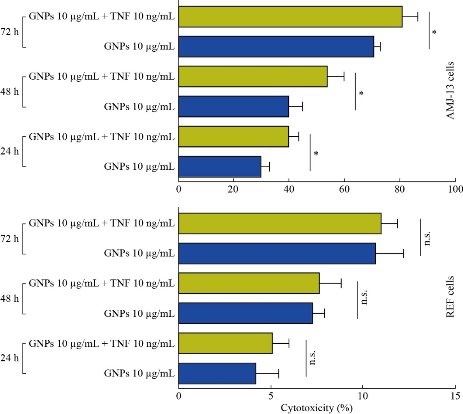
Fig. 4 Cytotoxicity and antiproliferative activity of GNP and TNF-α loaded on GNPs in AMJ13 and REF cell lines. The values represent mean ± SD.
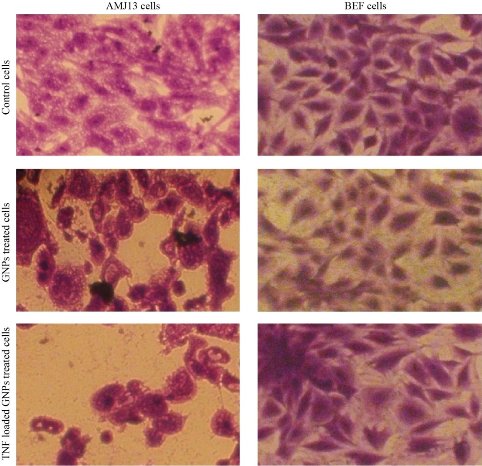
Fig. 5 Morphological changes in cells after being treated with GNP and TNF-α loaded on GNPs.
TNF-α loaded on GNPs inducing apoptosis in AMJ13 cells
Apoptosis is primarily characterized by biochemical pathways that depend on energy and morphological properties that are unique. Cells undergoing early stages of apoptosis characteristically exert chromatin condensation pyknosis as well as cell shrinkage, with the former being the most crucial event and the latter being a consequence of condensed organelles and dense cytoplasm. The changes in the nuclear morphology of AMJ13, after treated with GNPs (10 µg/mL), TNF-α loaded GNPs (10 µg/mL – 10 ng/mL), were studied by DAPI staining method. The apoptotic cells were evaluated based on DNA damage. The nuclear morphology of the cells was observed using DAPI staining. The results revealed condensation of the chromatin in the treated cells as opposed to normal morphology of the nucleus in the control ones. The results of this study showed that the proportion of apoptotic cells increased significantly in GNPs, and TNF-GNPs treated cells were compared with the untreated AMJ13 cells [31, 32]. To confirm our results, we measured the percentage of apoptotic AMJ13 cells after being treated with GNPs and TNF loaded-GNPs using a flow cytometer by staining of treated and untreated AMJ13 cells with annexin V-FITC. A sizable increase of apoptosis due to the treatment with GNPs and TNF loaded-GNPs in AMJ13 cells was clearly showed in Fig. 6. Our results showed that the percentage of apoptotic cells after treated with GNPs and TNF loaded-GNPs increased significantly compared with the control group of untreated AMJ13 cells.
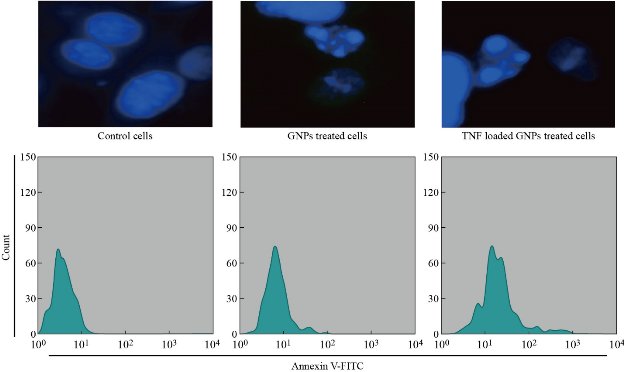
Fig. 6 GNPs and TNF-GNPs induced apoptosis of AMJ13 cells stained with DAPI as in upper panel and stained with Annexin V as in lower panel.
Effects of TNF-α loaded on GNPs on mitochondrial function
The mitochondria take a critical part in the induction of the apoptotic events through different stimuli of cellular death. Alterations in this organ are represented by the loss of its membrane potential (∆ψm) and the secretion of cytochrome c to the cytoplasm, resulting in the stimulation of caspase-3 through a caspase-9 pathway. Thus, we measured the effect of GNPs and TNF-GNPs on the ∆ψm in AMJ13 cells. The cells were treated with GNPs and TNF-GNPs for 24 h, followed by staining with a sensitive dye (JC-1) for probing the membrane potential of the mitochondria, and examination with the fluorescent microscope. AMJ13 cells were treated with GNPs, and TNF-GNPs caused higher percentage of cells, emitting red fluorescence as related to that in the control group (Fig. 7).
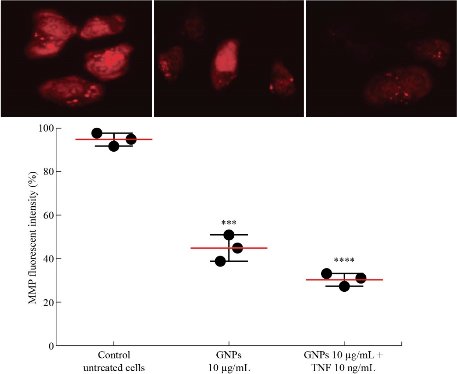
Fig. 7 GNPs and TNF loaded on GNPs reduced MMP of AMJ13 cells.
Detection of gene alteration using quantitive real time PCR (qRT-PCR)
In this study, quantitative real-time PCR was used to identify the change in the expression of mRNA of apoptotic gene p53 in AMJ13 cells that were exposed to GNPs, and TNF was loaded on GNPs for 24 h at concentrations of 10 µg/mL – 10 ng/mL. The PCR results showed that apoptotic markers at the mRNA level were altered with cell lines due to applying the GNP-TNF-α. The mRNA level of tumor suppression gene p53 showed an increase as shown in Fig. 8. The tumor suppressor p53 gene and caspase enzyme controlled the cell proliferation and prevented them from becoming cancerous [33]. If a cell noticed any kind of malignancy, a DNA repair mechanism was activated to restore the altered DNA [31]. TNF-α induced extrinsic apoptosis by binding to type I receptor (TNFRI), which would lead to activate caspase-dependent cell death [29], Activated TNFRI recruited TRADD (TNFR-associated death domain), which would stimulate the TRADD complex containing FADD (FAS-associated death domain) and pro-caspase 8, leading to the activation of caspase 8 and the initiation of an apoptotic signaling cascade [34, 35]. TNF-α also caused the accumulation of P53 in cancerous cell line that led to DNA fragmentation and cell death [36].
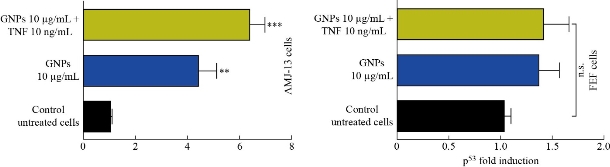
Fig. 8 GNPs and TNF up-regulated p53 expresiopn in AMJ13 and REF cell lines.
Toxicity of GNPs and TNF-α loaded on GNPs in vivo
In this study, body weight of injected animals was measured in mice following the intraperitoneal (i.p.) injection of GNPs and TNF-α loaded on GNPs for 4 weeks. The results revealed the absence of significant changes in the animal’s body (Fig. 8).
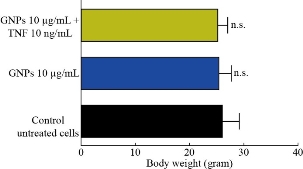
Fig. 8 Effect of GNPs, and TNF loaded on GNPs in body weight of mice (in-vivo) model
Conclusions
This study showed an effective way of binding the TNF-α to pegylated gold nanoparticles. The binding method represented an easy and less energy-dependent approach of producing well-defined anti-cancer drug. In this study, GNP-TNF-α was found to have a great potential to be considered as an anticancer drug delivery system. Similarly, GNP-TNF-α was remarkably more cytotoxic on the studied cell line than GNPs alone. The novelty of our work was to use GNPs and TNF-loaded GNPs as anticancer agents against Iraqi breast cancer cell line AMJ13 and to study the possible mechanisms of this effect, which involved mitochondrial damage and upregulation of p53 pathway. The results suggested that these compounds should be further investigated for potential application as cancers drug.
Conflict of interest
No potential conflict of interest was reported by the authors
Refrences
Copyright© Noor Abood, Majid Jabir, and Haider Kadhim. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.