Research Article
Spectrophotometric Determination of Cobalt(II) and Lead(II) Using (1,5-Dimethyl-2-Phenyl-4-((2,3,4-Trihydroxy Phenyl) Diazenyl)-1H-Pyrazol-3(2H)-One) as Organic Reagent: Using It as Antimicrobial and Antioxidants
Shaimaa Mohsen. Essa *, Wisam Hindawi Hoidy
Department of Chemistry, College of Education, University of Al-Qadisiyah, Al-Qadisiyah, Iraq.
* Corresponding author. E-mail: shaimaa.essa@qu.edu.iq
Received: Mar. 27, 2020; Accepted: Apr. 17, 2020; Published: Jun. 3, 2020
Citation: Shaimaa Mohsen. Essa, Wisam Hindawi Hoidy, Spectrophotometric Determination of Cobalt(II) and Lead(II) Using (1,5-Dimethyl-2-Phenyl-4-((2,3,4-Trihydroxy Phenyl) Diazenyl)-1H-Pyrazol-3(2H)-One) as Organic Reagent: Using It as Antimicrobial and Antioxidants. Nano Biomed. Eng., 2020, 12(2): 160-166.
DOI: 10.5101/nbe.v12i2.p160-166.
The azo organic reagent (1,5-dimethyl-2-phenyl-4-((2,3,4-trihydroxy phenyl) diazenyl)-1H-pyrazol-3(2H)-one) (DPTPD) was prepared and used for the spectrophotometric determination of cobalt(II) and Lead(II), by the selective and surfactant-sensitized method based on the ternary complexes formation of Co(II) and Pb(II). The reagent had absorption maximum at 381 nm, and reacted with Co2+ to form a purple reddish complex with λmax = 430 nm at pH = 7.5, while it formed a red complex with Pb2+ of λmax = 417 nm at pH= 6. Beer ҆s law for the determination over the range of 1 - 25 ppm and 1 - 33 ppm for Co(II) and Pb(II), respectively. The molar absorptivity (Є) and Sandell’s sensitivity values of Co(II) and Pb(II) complexes were found to be 1.02 × 104, 3.3 ×104 mol-1 cm-1, and 0.0725, 0.0269 μg cm-2 at 430, 417 nm, respectively. The stability constant was found to be 1.1 × 108 L mol-1 and 2.3 × 108 L mol-1 for Co(II) and Pb(II), respectively. Detection limit relative standard deviation, relative error and recovery were predestined for 15 ppm standard solution of Co(II) and Pb(II) complexes, respectively. The important interferences with most ions like Cr+3, Mn2+, Fe3+, Zn2+, Hg2+, Mo+2, Pt2+ and Cd+2 were studied using the appropriate masking agents. The method was applied for the determination of Co(II) in filling sample; the result obtained was incompatible with that by the flame atomic absorption spectrometry method. The organic reagent (DPTPD) was diagnosed as an anti-bacterial and an antioxidant.
Keywords: Heterocyclic; Pyrazol; Azo; Antipyrene; Antimicrobial; Antioxidants
Introduction
Antipyretics were synthesized in 1884, and through research and studies this organic compound was discovered to be a pain reliever. By 1930, it had become one of the most important analgesics in the pharmaceutical world. With time passing by, antipyretics have proven to be of great value among pharmaceuticals [1, 2]. In this study, the heterocyclic azo dyes were synthesized and proposed as a highly sensitive chromogenic reagent for the determination of several metal ions [3, 4]. The chemical compound (1,5-dimethyl-2-phenyl-4-((2,3,4-trihydroxy phenyl) diazenyl)-1H-pyrazol-3(2H)-one) was prepared [5] and used as organic reagent and antioxidant compound in this study. Cobalt is an element of high industrial importance in alloying, coating and dyeing. It is used as a catalyst because of its important properties; it is a transitional element and also has biological usages as this element can be an active center of auxiliary enzymes, for example vitamin B (cyanocobalamin), which is used as radiation therapy for cancer patients [6, 7]. Lead is a major cause of many human killer diseases, leading to dysfunction of blood and nervous system. Lead can also precipitate easily in the brain, kidneys and reproductive system. Another popular disease is lead poisoning, which leads to anemia and brain damage and ultimately to death [8]. Several techniques such as flame atomic absorption, voltammetric and spectrophotometric methods have been used for determination of the ion in different samples, among which ultraviolet-visible spectrophotometry (UV-Vis) is the most commonly used technique for Pb(II) [9]. There are many studies conducted to determine the elements; however, our research for the first time completed the estimation of cobalt(II) and lead(II) using (1,5-dimethyl-2-phenyl-4-((2,3,4-trihydroxy phenyl) diazenyl)-1H-pyrazol-3(2H)-one) as organic reagent. On the other hand, this work has been applied in 2 ways: The first with regard to the reagent in a field of antioxidant, and the second concerning determination of cobalt in the filling of teeth.
Experimental
Apparatus
Absorption spectra in absolute ethanol were recorded using UV-Vis spectrophotometer T80, England, using 1 cm quartz cells. Functional groups of reagent and its complexes were identified using Fourier-transform infrared (FTIR) spectrometer Shimadzu 8400, in the range of 4000 - 400 cm-1 using KBr disc. pH measurements were carried out using a Philips PW 9421 pH meter (pH 0.001), AA-6300 atomic absorption spectrophotometer, Pg, Japan.
Reagents and solutions
All chemicals used were of high purity without additional purification. Ethanol was purchased from GCC, England. 5-sulfosalicylic acid, NaNO2, sodium acetate, oxalic acid, ascorbic acid, tartaric acid, Na2S2O3, KCl and KI were purchased from Sigma-Aldrich. The reagent 1 × 10-4 mol L-1 (1,5-dimethyl-2-phenyl-4-((2,3,4-tri-hydroxy phenyl) diazinil)-1H-pyrazol-3 (2H)-one) was prepared for dissolving route of reagent in 250 mL absolute ethanol. Co(II) and Pb(II) ion solutions (1000 mg L-1) were prepared by dissolving 0.4033 g of CoCl2.6H2O (BDH) and 0.1598 g of Pb(NO3)2 in 100 mL deionized water, standard solutions for each metal were made by appropriate dilutions.
Antimicrobial study
In a standard 10 mL conical flask, 1.5 mL of Co(II) solution containing less than 100 gm mL-1 of it was transferred, where the pH was adjusted to 7.5 using ammonium acetate solution; then 2 mL 1.0 × 10-4 M of ethanolic reagent was added and diluted with deionized water to the marker. At 430 nm and 25 ℃, the resulting solution absorption was measured after 10 min. In the same way, a solution was prepared for a lead(II) element but with a pH = 6, and the absorbance was measured at 417 nm after 10 min. The antibacterial tests were performed according to the disc diffusion method. (1,5-dimethyl-2-phenyl-4-((2,3,4-trihydroxy phenyl) diazenyl)-1H-pyrazol-3(2H)-one) (DPTPD) was examined for its antimicrobial action in vitro against 4 strains of bacteria, 2 of which were gram-positive and 2 gram-negative represented by Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Klebsiella pneumoniae, respectively. In addition, single fungal - Candida albicans was used; amoxicillin and cephalexin were utilized as a positive control and DMSO was employed as a negative control; nystatin was employed as antifungal reference drug. Bacteria were incubated and cultured at 37 °C for 24 h. To prepare media, agar was poured into the sterilized petri discs. After that, the cultures were scattered on the surface of nutrient agar (NA). Antimicrobial action was defined by measuring the diameter of inhibition zone (IZ) in comparsion with positive control where the concentration of all compounds was 30 μg mL-1.
Antioxidants study
To clarify whether the reagent (1,5-dimethyl-2-phenyl-4-((2,3,4-trihydroxy phenyl) diazenyl)-1H-pyrazol-3(2H)-one) had the potential to act as an antioxidant. The plasmid was first extracted from E. coli bacteria according to the extraction method of the kit; then the electrophoresis for the samples was done. The extract was good and gave clear bands.
Results and Discussion
Infrared spectra
The IR spectrum of the reagent showed absorption at 1710 cm-1 for C=O, 1504 cm-1 for -N=N-, 3455 cm-1 for OH of phenol, and showed band at 3088 cm-1 for aromatic C-H and band at 2994 cm-1 for aliphatic C-H. The presence of new medium intensity bands in the 420 - 455 and 480 - 520 cm–1 regions, assignable to ʋ(M–N) and ʋ(M–O) in the spectra of all complexes [10, 11] as shown in Table 1.
Table 1 Important IR frequencies for the ligand and its complexes
|
Compound |
ʋ(O-H) |
ʋ(N=N) |
ʋ(C=O) |
ʋ(Ar-H) |
ʋ(M-O) |
ʋ(M-N) |
|
Reagent |
3380 |
1504 |
1710 |
3067 |
-- |
-- |
|
Co-complex |
3329 |
1572 |
1664 |
3066 |
488 |
455 |
|
Pb-complex |
3388 |
1563 |
1711 |
3088 |
501 |
401 |
Absorption spectra and characteristics of the complexes
UV-Vis for the ethanolic solution of the reagent (1 ×10-4 M) showed 3 clear peaks. The first and the second peaks were at 279 nm and 239 nm and indicated the transition of -π* of the aromatic rings. The third peak was at 381 nm and attributed to π-π* of the aromatic ring through the azo group. Transfer of charge referred to the transmission of n- of * to charge the intermolecular charge place of benzene through the azo group -N=N [12]. In aqueous ethanol solution, the reaction of metal ions Co(II) and Pb(II) with the reagent was studied. A bathochromic shift of Co(II) and Pb(II) complexes showed the absorption maxima of 430 and 417 nm with molar absorptivities (ε) as of 1.02 × 104 L mol-1 cm-1 and 3.3 × 105 L mol-1 cm-1, respectively. The reagent gave the absorption maxima of 381 nm as depicted in Fig. 1. The wavelength difference Δλmax was 49-36 nm; a great bathochromic shift in the visible region was detected in the complex solution’s spectra concerning that of the free reagent. The high shift in the λmax gave a good indication for complex formation.
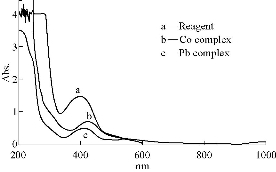
Fig. 1 Absorption spectra of (a) reagent = 1.0 × 10-4 M, (b) Co(II)-complex (15 ppm) at pH = 7.5, and (c) Pb(II)-complex (15 ppm) at pH=6.
Method validation
Under optimal conditions, graphs were designed based on optimal calibration conditions and were done by drawing the absorption signal versus the concentrations of each analytical substance. In the 10 mm optical cell, solutions were placed to measure the spectral metal ion at maximum absorption. The calibration data is shown in Table 2.
Table 2 Method validation of the spectrophotometric determination of Co(II) and Pb(II)
|
Pb(II) |
Co(II) |
Parameter |
|
417 |
430 |
λmax (nm) |
|
y = 0.0372x + 0.1115 |
Y=0.0138X+0.0119 |
Regression equation |
|
0.9947 |
0.9975 |
Correlation coefficient(r) |
|
1-33 |
1-25 |
Concentration range (μg mL-1) |
|
0.3 |
0.241 |
Limit of Detection (μg mL-1) |
|
0.0269 |
0.0725 |
Sandell's sensitivity (µg cm-2) |
|
1.02 × 104 |
3.3×104 |
Molar absorptivity (L mol-1 cm-1) |
|
1:2 |
1:2 |
Composition of complex (M: L) |
|
2.6 |
2.1 |
RSD% (n = 7) at 15 μg Co(II) mL-1 and 15 μg Pb (II) mL-1 |
|
97.54 |
99 |
Recovery% |
Effect of pH
The effect of pH on the formation of Co(II) and Pb(II) complexes was determined by recording their absorbance signals at λmax, over the range of 3-9, using different pH acetate buffer solutions. The results are described in Fig. 2. From Fig. 2, it can be seen that the absorption increased with the increase of pH, where absorption increased and reached the maximum at pH 7.5 and 6 for Co(II) and Pb(II) complexes, respectively. A gradual decrease in absorbance was then observed due to the partial disintegration of complexes at high pH. From all these observations, pH 7.5 and 6 were selected as the best pH for the formation of Co(II) and Pb(II) complexes, respectively.
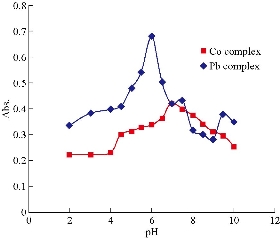
Fig. 2 Effect of pH on complexes formed with Co(II) and Pb(II). Conditions: Co(II) = 15 μg mL-1, and 2 mL of the reagent = 1.0 × 10-4 M; Pb(II) = 15 μg mL-1, and 2 mL of the reagent = 1.0 × 10-4 M.
Effect of temperature and time
The effect of temperature and time was studied for their importance in completing the reaction. Experiments were conducted to determine the temperature range at which the high absorption signals of Co(II) and Pb(II) were apparent. Temperatures between 20 and 70 ℃ were found to have the optimum absorption value. The best temperature was 40 ℃, where the highest absorption signal is shown in Fig. 3. It was also observed that the incubation time of 10 min was sufficient for the maximum absorbance of Co(II) and Pb(II), as shown in Fig. 4.
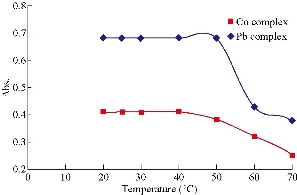
Fig. 3 Effect of temperature on the absorbance of complexes formed with Co(II) with 2 mL of the reagent = 1.0 × 10-4 M, and Pb(II) = 15 μg mL-1 with 2 mL of the reagent = 1.0 × 10-4 M.
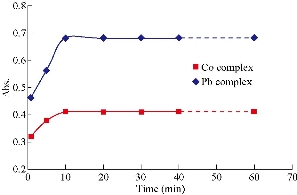
Fig. 4 Effect of time on the absorbance of complexes formed with Co(II) and Pb(II). Conditions: Co(II) = 15 μg mL-1, 2 mL of the reagent = 1.0 × 10-4 M, and Pb(II) = 15 μg mL-1, 2 mL of the reagent = 1.0 × 10-4 M.
Composition and stability of complexes
The composition of chelate complexes was determined by mole ratio and continuous variation method (Fig. 5 and 6). Both methods showed that the molar ratio of Co(II) and Pb(II) ions to reagent was 1 : 2, and the suggested related chemical structures are shown in Fig. 7. The stability constant was found to be 1.1 × 108 L mol-1 and 2.3 × 108 L mol-1 for Co(II) and Pb(II), respectively.
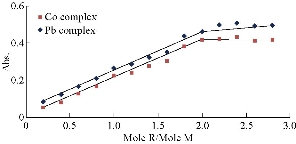
Fig. 5 Mole ration method for Co(II)-complex at pH = 7.5 and Pb(II)-complex at pH = 6.
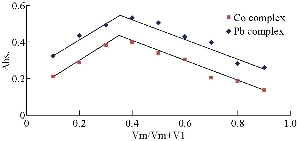
Fig. 6 Continuous variation method for Co(II)-complex at pH = 7.5 and Pb(II)-complex at pH = 6.
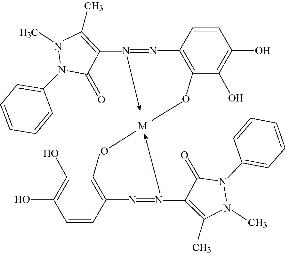
Fig. 7 Proposed structural formula for the complexes (M = Co(II) and Pb(II) ion).
Interference studies of cobalt(II) and lead(II) complexes
The effect of the interference of ions which formed complexes with the reagent (1,5-dimethyl-2-phenyl-4-((2,3,4-trihydroxy phenyl) diazenyl)-1H-pyrazol-3(2H)-one) during its reaction with cobalt and lead (15 ppm) was studied. The selectivity of the various masking agents was examined for eliminating the effect of each of the interfering 8 ions. These were 5-sulfosalicylic acid, NaNO2, sodium acetate, oxalic acid, ascorbic acid, tartaric acid, Na2S2O3, KCl and KI. The results are shown in Table 3 and 4.
Table 3 Effect of exotic ions on the determination of Co(II) and in concentration (15) ppm and suitable masking agents
|
Foreign ion 100 ppm |
Masking agent (1.0) mL [0.01] M |
Error% |
|
Cr+3 |
5-Sulfosalicylic (0.5) [0.01] |
0.003 |
|
Mn2+ |
NaNO2 (0.2) [0.1] |
0.002 |
|
Fe3+ |
5-Sulfosalicylic (0.75) [0.01] |
0.004 |
|
Zn2+ |
Sodium acetate (0.5) [0.01] |
-0.001 |
|
Hg2 |
Oxalic acid (0.4) [0.2] |
-0.002 |
|
Mo+2 |
Oxalic acid (0.4) [0.2] |
-0.004 |
|
Pt2+ |
Sodium acetate (0.5) [0.01] |
-0.001 |
|
Cd+2 |
5-Sulfosalicylic (0.75) [0.01] |
0.001 |
|
Pb+2 |
Ascorbic acid |
0.001 |
Table 4 Effect of exotic ions on the determination of Pb(II) in concentration 15 ppm and suitable masking agents
|
Foreign ion 100 ppm |
Masking agent (1.0) mL [0.01] M |
Error% |
|
Cr+3 |
KCl (0. 5) [0.02] |
0.003 |
|
Mn2+ |
Ascorbic acid (0.3) [0.1] |
0.002 |
|
Fe3+ |
5-Sulfosalicylic(0.75 )[0.01] |
0.004 |
|
Zn2+ |
KI (0.4) [0.01] |
-0.001 |
|
Hg2 |
Tartaric acid (0.5) [0.3] |
-0.002 |
|
Mo+2 |
Oxalic acid (0.4) [0.2] |
-0.004 |
|
Pt2+ |
Sodium acetate (0.5) [0.01] |
-0.001 |
|
Cd+2 |
5-Sulfosalicylic (0.75) [0.01] |
0.001 |
|
Co+2 |
Na2S2O3 (0.05) [0.01] |
0.001 |
Application
The proposed method was applied to the determination of cobalt in filling. The results are shown in Table 5. The obtained average of the 3 determinations was compared with that given by the atomic absorption spectrophotometer (AAS) (standard addition method).
Table 5 Determination of cobalt in filling
|
Standard sample |
Certified content (%) |
Proposed method (%)* |
|
Rua Funchal 376** |
3.30 |
3.05±0.13 |
* Average of 3 determinations, at 95% confidence level.
** Provided from Degussa Dental Ltd., Brasil. Sample Composition Ag (71%), Sn (25.7%), and Co (3.3%).
Antimicrobial actions
The antibacterial and antifungal actions of (1,5-dimethyl-2-phenyl-4-((2,3,4-trihydroxy phenyl) diazenyl)-1H-pyrazol-3(2H)-one) (DPTPD) was examined against 2 samples of gram-positive bacterias and 2 samples of gram-negative bacteria, i.e. Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Klebsiella pneumoniae, respectively, as well as single fungal Candida albicans utilizing disc diffusion method. Amoxicillin and cephalexin were used as positive controls; nystatin was used as positive control for fungal (concentration of 30 μg mL-1 for all of these compounds). The outcomes are itemized in Table 6 and 7. The antibacterial activity of (1,5-dimethyl-2-phenyl-4-((2,3,4-trihydroxy phenyl) diazenyl)-1H-pyrazol-3(2H)-one) was higher than amoxicillin but lowers than cephalexin because (1,5-dimethyl-2-phenyl-4-((2,3,4-trihydroxy phenyl) diazenyl)-1H-pyrazol-3(2H)-one) contained 4 atoms of nitrogen which increased the lipophilicity of this compound, while amoxicillin had only 1 atom of sulfur in its structure. These features displayed that the compounds generally enhanced permeability towards gram-positive and gram-negative bacteria cell tissue. It was identified that (1,5-dimethyl-2-phenyl-4-((2,3,4-trihydroxy phenyl) diazenyl)-1H-pyrazol-3(2H)-one) compound showed good antimicrobial actions in general.
Table 6 Antimicrobial result for (1,5-dimethyl-2-phenyl-4-((2,3,4-trihydroxy phenyl) diazenyl)-1H-pyrazol-3(2H)-one) compound
|
Compounds (30 mg mL-1) |
Inhibition zone (mm) |
|||
|
Gram-positive |
Gram-negative |
|||
|
Staphylococcus aureus |
Bacillus subtilis |
Escherichia coli |
Klebsiella pneumoniae |
|
|
DPTPD |
17.3 |
16.7 |
17 |
18.7 |
|
Amoxicillin |
14.4 |
13.4 |
15.1 |
12.9 |
|
Cefalexin |
18.2 |
16.3 |
18.1 |
18.6 |
|
DMSO |
-- |
-- |
-- |
-- |
Table 7 Inhibition zones of (1,5-dimethyl-2-phenyl-4-((2,3,4-trihydroxy phenyl) diazenyl)-1H-pyrazol-3(2H)-one) and the references antifungal
|
Compounds (30 mg mL-1) |
Inhibition Zone (mm) |
|
Candida albicans |
|
|
DPTPD |
19.6 |
|
Nystatin |
17.4 |
|
DMSO |
-- |
Antioxidants
The way to know the reagent's ability to act as an antioxidant is to add the Fenton reagent (which contains hydrogen peroxide, a strong oxidizer with catalysts, ferric chloride and ascorbic acid) to the plasmid, where the Fenton compound works on the oxidation of the plasmid, and the plasmid is destroyed. Hence, when the working electrophoresis of the plasmid completed oxidation, it does not give a single band but rather 2 or more bands, or the bands disappear permanently according to the degree of damage. In our experiment, 2 bands appeared, indicating the plasmid was split into 2 parts due to the oxidizing factors in the Fenton reagent, as shown in Fig. 8 (locations 2 and 4). On the other hand, when adding the compound (1,5-dimethyl-2-phenyl-4-((2,3,4-trihydroxy phenyl) diazenyl)-1H-pyrazol-3(2H)-one) to tubes 1 and 3, it appeared that the reagent Fenton did not affect the plasmid as the compound acted as an antioxidant, interacted with the Fenton reagent, and canceled its susceptibility to oxidation. Therefore, the single band appeared at sites 1 and 3, which indicated the plasmid was not affected by the oxidizing agent due to the presence of compound (1,5-dimethyl-2-phenyl-4-((2,3,4-trihydroxy phenyl) diazenyl)-1H-pyrazol-3(2H)-one) working as antioxidants. These results are consistent with many studies such as those conducted by Razack et al. [13] and by Lahneche et al. [14].
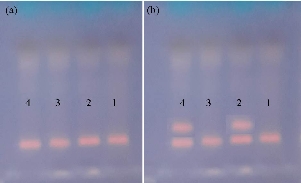
Fig. 8 (a) Electrophoresis of plasmid extracted from E. coli. (b) Plasmid electrophoresis image after plasmid (0.5 µg) was used with Fenton reagent (30 mM H2O2, 50 µM ascorbic acid, and 80 µM FeCl3). The compound (1,5-dimethyl-2-phenyl-4-((2,3,4-trihydroxy phenyl) diazenyl)-1H-pyrazol-3(2H)-one) added to model No. 1 and No. 3.
Conclusions
Synthesis and preparation of organic reagent were widely used in this study. We found that the reagent (1,5-dimethyl-2-phenyl-4-((2,3,4-tri-hydroxy phenyl) diazinil)-1H-pyrazol-3(2H)-one) reacted with elemental solutions of Co(II) and Pb(II), and that insoluble complexes were formed in water. The spectrum method was found to be simple, fast and uncostly, providing accurate results compared to the other methods, which makes it an alternative to the current methods of identifying these metal ions. On the other hand, this work has been applied in 2 ways: one with regard to the reagent in the field of antioxidants, and the other concerning the determination of cobalt in the filling of teeth. By conducting tests on the reagent as an anti-oxidant and anti-bacterial agent, it was found that the reagent was highly effective as an anti-oxidant through the results that it showed in protecting the plasmid from oxidation by oxidizing agents. The organic compound also showed high efficacy as an anti-bacterial when its effect was compared with known antibiotics.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Shaimaa Mohsen. Essa, Wisam Hindawi Hoidy. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.