Review
Revisiting an Old Friend Nano-TiO2: A Crucial Assessment of its Current Safety
Ozioma Udochukwu Akakuru 1, 2, Muhammad Zubair Iqbal 1, 3*, Chuang Liu 1, 2, Zihou Li 1, 2, Yang Gao 1, Chen Xu 1, Elvis Ikechukwu Nosike 1, 2, Gohar Ijaz Dar 1, 2, Fang Yang 1, Aiguo Wu 1*
1 Cixi Institute of Biomedical Engineering, CAS Key Laboratory of Magnetic Materials and Devices, & Key Laboratory of Additive Manufacturing Materials of Zhejiang Province, Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, Ningbo 315201, P.R. China.
2 University of Chinese Academy of Sciences, No.19(A) Yuquan Road, Shijingshan District, Beijing, 100049, P.R. China.
3 School of Materials Science and Engineering, Zhejiang Sci-Tech University, No. 2 Road of Xiasha, Hangzhou, 310018, PR China.
*Corresponding authors. E-mail: aiguo@nimte.ac.cn; zubair@nimte.ac.cn Tel: +86 574 87617278 Fax: +86-57486685163
Received: Oct. 26, 2019; Accepted: Feb. 7, 2020; Published: Feb. 14, 2020
Citation: Ozioma Udochukwu Akakuru, Muhammad Zubair Iqbal, Chuang Liu, Zihou Li, Yang Gao, Chen Xu, Elvis Ikechukwu Nosike, Gohar Ijaz Dar, Fang Yang, and Aiguo Wu, Revisiting an Old Friend Nano-TiO2: A Crucial Assessment of its Current Safety. Nano Biomed. Eng., 2020, 12(1): 21-46.
DOI: 10.5101/nbe.v12i1.p21-46.

Ozioma Udochukwu Akakuru obtained his Masters Degree in Industrial Chemistry from the University of Benin, Nigeria in 2013. He then joined the Department of Chemistry, University of Calabar, Nigeria in 2014 as a lecturer. In 2017, he was awarded the CAS-TWAS President's Fellowship for a Doctorate Degree at Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, Ningbo, China. He has more than 30 publications in scientific journals of international repute. His research focuses on the synthesis and application of targeted nanomaterials for image-guided cancer therapy.

Muhammad Zubair Iqbal received his PhD in Experimental Condensed Matter Physics from University of Science and Technology Beijing in 2013. Then he joined the Ningbo Institute of Materials Technology and Engineering (NIMTE), Chinese Academy of Sciences (CAS) as a postdoctoral fellow under the supervision of Prof. Aiguo Wu. He is currently working as an Associate Professor in School of Materials Science and Engineering at Zhejiang Sci-Tech University. His research focuses on the design and synthesis of nanomaterials for biomedical applications Such as molecular imaging contrast agents, targeting drug delivery systems, and phototherapy of cancers. He has published 90+ articles in international reputed journals.

Aiguo Wu got his bachelor degree from Department of Chemistry in Nanchang University in China and received his PhD from Chinese Academy of Sciences supervised by Prof. Erkang Wang and Prof. Zhuang Li at State Key Laboratory of Electroanalytical Chemistry in Changchun Institute of Applied Chemistry, China in 2003. He ever stayed in University of Marburg (Prof. Norbert A. Hampp group) in Germany during 2004-2005, California Institute of Technology (Prof. Ahmed Zewail group) in USA during 2005-2006 and Feinberg School of Medicine in Northwestern University (Prof. Gayle E. Woloschak group) in Chicago, USA during 2006-2009. In 2015, he came back University of Marburg, Germany as a visiting Professor again. In 2009, he joined Ningbo Institute of Materials Technology & Engineering (NIMTE), Chinese Academy of Sciences (CAS) as a PI. Prof. Wu has published over 190 papers in peer review journals, four books and seven book chapters, and awarded 61 invention patents. His lab focuses on using nanoprobes for early diagnosis, imaging, therapy and theranostics of diseases, particularly in cancer etc.
Abstract
The recent advances in nanoscience and nanotechnology have enhanced the synthesis of various forms of nanomaterials for practical applications. Undoubtedly, these nanomaterials are not without attendant toxicities to humans, environment, and other organisms. Moreover, the toxicity of nanomaterials dominates the landscape of current toxicity concerns highlighted by the FDA. Titanium dioxide nanoparticles (TiO2 NPs) contribute a large proportion of synthesized nanomaterials mainly due to their excellent photocatalytic activities, mechanical and chemical stability, bio- and chemical inertness, corrosion resistance, thin film transparency, and low production cost. These fascinating properties of TiO2 NPs have been extensively exploited and dramatically increased their utility for various applications such as in nanomedicine for cancer theranostics, nanobiotechnology, environment, pharmacy, energy, food, cosmetics, and paper industries. Owing to the poor understanding of the impacts of NPs on humans, no clear regulation has been implemented for NPs among international authorities. Over time, the toxicity state of TiO2 NPs is typical of a double-edged sword. Hitherto, there is no restriction on the use of TiO2 NPs irrespective of the toxicity concerns raised by some researchers. This may have been dampened by the low-to-no toxicity reports from other researchers on these NPs. This review therefore looks into the recent toxicity reports from various studies conducted with/on TiO2 NPs as to ascertain their present-day safety. To elucidate this, we discussed the possible exposure routes to these NPs and their effects on the environment, plants, soil organisms, and aquatic species. We also provided insights on the toxicity mechanisms of TiO2 NPs and proposed future perspectives for improving their safe applications.
Keywords: Exposure routes; Environmental fates; TiO2 nanoparticles; Toxicity mechanism
Introduction
The toxicity of nanomaterials is at the fore-front of recent toxicity concerns highlighted by the Food and Drug Administration (FDA) of the United States of America. This follows the discovery that some nanomaterials which were earlier approved for clinical use contain some amounts of carcinogenic substances [1-3]. Particularly, the toxicity of titanium dioxide nanoparticles (TiO2 NPs) is an important issue in present-day toxicology [4-6]. Owing to their excellent near infrared reflective and electrical properties, TiO2 NPs have achieved a wide range of applications, necessitating an assessment of their current toxicity. TiO2-based NPs have been specifically used in cosmetics [7], phamaceutical products [8, 9], food colouration [10], plastic products [11, 12], antifouling agents marine paints [13], photocatalysis and microbial boosting for soil remediation [7, 14], filters [15], bacterial treatment [16], and in the photothermal [17, 18], photodynamic and sonodynamic ablation of cancers [19, 20]. Many other new applications of TiO2 NPs are underway or possibly in pilot production, making TiO2 NPs and their derivatives to be ranked among the most commonly-produced nanomaterials world wide [7]. Based on the afore-mentioned extensive applications of TiO2 NPs and their inclusion in commercial products, human exposure to these NPs and their derivatives either during production or end use is inevitable. A Recent study showed that even candies, sweets and bubble gums contain high amounts of TiO2 NPs (˂100 nm) [10]. When NPs enter the body, they are usually transported to various systems via systemic circulation and are thereafter deposited in tissues or organs, causing toxicity [21, 22]. TiO2 NPs with size ranges between 5–100 nm can easily enter into rat lungs and then lymphatic drainage and blood vessels. They may also systemically reach the central-nervous and immune systems as well as the cardiovascular system, with the potential of being toxic if not eliminated from the body [23]. Nano-sized TiO2 has been reported to cause redox imbalance by way of increasing the production of reactive oxygen species (ROS) or by inactivating/ inhibiting the components of cellular antioxidant defence system [24]. The induction of oxidative stress by TiO2 NPs following mechanistic toxicological investigations has been reported to predominantly cause cell damage, impaired immune response, inflammation and genotoxicity [25]. In this paradigm, Gao and co-workers on the testicular damage and alterations in gene expression profiles in male mice caused by intragastric administration of TiO2 NPs [26]. It was observed that NPs crossed the blood-testis barrier, got to the testis and resulted in testicular lesions, alterations in serum sex hormone levels, and sperm malformations. These researchers therefore inferred that the production and application of TiO2 NPs should be with caution, particularly when the persons are of reproductive age. Up till now, toxicological studies with TiO2 NPs have been carried out mostly in mammals, animal models (mice) or small organisms such as Daphnia (a fish species) [5]. These studies reveal that the type and extent of damage by TiO2 NPs are strongly dependent on the physical and chemical characteristics of the NPs, which govern their reactivity and bioavailability [25]. For instance, the isoelectric points for TiO2 NPs range from pH 3.5 to 8, which may greatly affect the bioavailability of these NPs in the physiological environment [27]. Unfortunately, the effective size of particles, concentrations and their Zeta-potentials have been almost completely neglected in most of the studies on interaction of TiO2 NPs with biological systems [25, 27]. It has also been reported that upon UV irradiation, TiO2 NPs exhibit photoactivities which have been explored for numerous applications. It is rather worrisome that little attention has been focused on the toxic effects of these NPs upon UV irradiation, mainly to non-target organisms. Recent reports on the toxicity of these photoactivated TiO2 NPs have raised serious concerns among researchers as their long-term toxicity effects cannot also be ascertained [7]. Owing to the wide applications of TiO2 NPs and the ever-increasing synthesis of pristine and modified TiO2 NPs, we believe that a review of their current toxicity is paramount. Undoubtedly, this will benefit various researchers in this field, the government, and other agencies concerned with the toxic effects of NPs. This review will therefore discuss the recent findings on the potential exposure hazards of TiO2 NPs to animal models, humans, and the environment by extension, with respect to the exposure routes and toxicity mechanisms of the NPs.
Exposure Routes to TiO2 NPs
There are numerous routes through which humans and animal models are exposed to TiO2 NPs. Among these, intravenous injection remains the most prominent, especially for animal models during the testing of synthesized TiO2 NPs for various applications in vivo. Detailed discussion on some of these exposure routes (intravenous, intraperitoneal, and subcutaneous injections, prenatal, dermal, body implants, oral, inhalation, and intratracheal instillation) will be discussed in the following sub-sections. However, other routes to the exposure of TiO2 NPs include but not limited to abdominal/intra-abdominal exposure, intragastric administration, and intra-articular injection. Meanwhile, the exposure routes to TiO2 NPs as well as their transportation and distribution sites can be summarized in Fig 1.
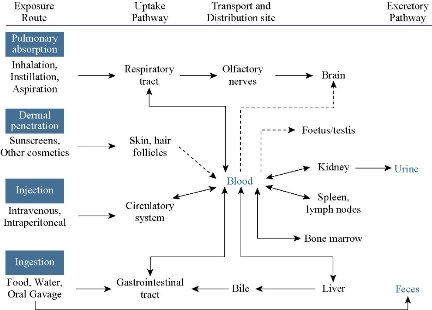
Fig. 1 Toxicokinetics and accumulation sites of TiO2 NPs. The dotted lines arrows represent uncertainties.
Intravenous injection
In nanomedicine, TiO2 NPs and their derivatives are directly injected intravenously into the blood vessels, thereby raising public concerns on the toxicity of these NPs to animals and their fate if translated to humans [28]. In this regard, Xu and co-workers investigated the acute toxicity of TiO2 NPs on mice induced by intravenous injection [29]. They observed that the spleen of the mice administered with the NPs demonstrated higher tissue weight/body weight coefficients and lower kidney and liver coefficients. Even though the mice hearts showed no pathological effects, there were some damages observed in the brain, liver, kidneys, lungs, and spleen of the mice, induced by the TiO2 NPs. Furthermore, they reported that low doses of the TiO2 NPs caused acute toxicity symptoms such as increased white blood cell count, decreased food and water intake, and physical activity, while the highest dose (1387 mg/kg body weight) caused death on the second day after intravenous injection. In a study by Yamashita and co-researchers on pregnant mice intravenously injected with 0.8 mg of 35 nm TiO2 NPs for two consecutive gestational days, pregnancy complications such as the accumulation of the NPs in the liver, brain and placenta of the foetus and smaller uteri and foetuses were observed [30]. In another study, it was reported that TiO2 NPs converted benign mouse fibrosarcoma cells to aggressive tumor cells [31]. The researchers intravenously injected female C57BL/6 mice with 5 mg/0.1 mL hydrophilic and hydrophobic rutile TiO2 NPs separately treated with ZrO2Al(OH)3 and observed both metastatic and carcinogenic abilities arising from tumour cell lines that were obtained from QR-32 cells. These QR-32 cells were reported to be tumorigenic following injection in sites pre-implanted with hydrophilic TiO2 NPs for 30 or 70 days. Also, van Ravenzwaay and others intravenously injected male Wistar rats with TiO2 NPs and monitored their behaviors up to 28 days [32]. They observed that these NPs mainly accumulated in the spleen and liver of the rats, subsequently causing an inflammation of these organs. This was explained by an increase in total cell count, lactate dehydrogenase, Γ-glutamyl transpeptidase, polymorphonuclears, ALP, total protein content, and N-acetyl-glucosaminidase in bronchoalveolar lavage. In a different experiment, TiO2 NPs also caused liver damage and significantly induced the expression of glutathione reductase in male Wistar rats which were injected intravenously with 5 mg/kg body weight of the NPs for 5 days [33]. Geraets and co-workers also observed the rapid distribution of free titanium through the systemic circulation in the heart, kidneys, liver, lungs, spleen, reproductive organs, thymus, and brain of mice following a single and repeated intravenous injection of TiO2 NPs [34]. The maximum relative decrease of 26% was observed during the 90-day post-exposure period. Inasmuch as little variations in kinetic profile were also observed among the various particles, these could not be clearly related to the hydrophobicity or the primary TiO2 NPs size differences. Furthermore, Iqbal and others intravenously injected mice with TiO2-Mn3O4 Janus NPs and monitored the accumulation of these NPs in the mice hearts, livers and kidneys up to 160 mins [35]. They observed that the Janus NPs showed low accumulation in the hearts but demonstrated maximum accumulation in the livers and kidneys between 30 to 50 mins and sharply declined at 60 mins. Su and co-researchers evaluated the toxicity of Janus Fe3O4-TiO2 NPs (20-25 nm) and the parent TiO2 NPs (7-10 nm) on Sprague Dawley rats following intravenous injection [19]. Extensive biosafety analyses (hematological, histopathological, biochemical, Western blot analysis, and element content) were conducted on the rats at 30 days post-injection of the NPs. The elemental analysis revealed slight accumulation of Ti in the hearts, livers and spleens of the rats treated with Fe3O4-TiO2 NPs. On the other hand, the rats treated with spherical shaped TiO2 NPs revealed significant accumulation of Ti in the lungs, livers and spleens. They observed that both the Janus Fe3O4-TiO2 and the parent TiO2 NPs have potential for causing histopathological abnormalities. These NPs were also found to have potential for inducing certain apoptotic or inflammatory-related molecular protein upregulation in the livers of the rats. Some alterations in the liver function to a certain degree were also observed. Additionally, at 30 mg/kg, the TiO2 NPs demonstrated much more sever adverse effects than the Janus counterparts. In a different experiment, Ren and co-workers intravenously injected Blab/c mice with various doses (1, 5, 25 mg/kg body weight) of hydrogenated black-TiO2 NPs functionalized with PEG (H-TiO2-PEG) and monitored the drinking, eating, activity, excretion and nuerological status of the mice models [20]. After one month post intravenous injection, no obvious tissue damage or lesions including necrosis, pulmonary or inflammatory fibrosis was observed (Fig. 2(a)). Additionally, they analysed the red blood cell (RBC), white blood cell (WBC), platelet (PLT), and their relevant data such as lymphocyte (LY), neutrophil (NE), monocyte (MO), basophil (BASO), hematocrit (HCT), eosinophil (EO), hemoglobin (HGB), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCHC), platelet distribution width (PDW-CV), red blood cell distribution width (RDW-CV), and mean platelet volume (MPV). No obvious variation was observed in the blood hematological analysis of the control and H-TiO2-PEG-injected mice groups (Fig. 2(b)). Six important hepatic indicators for liver functions (albumin, ALB; direct bilirubin, DBIL; alkaline phosphatase, ALP; globin, GLOB; gamma glutamyl transpeptidase, GGT), three indicators for kidney functions (creatinine, CREA; urea nitrogen, UREA; uric acid, URCA), triglyceride (TG), total cholesterol (CHOL), and glucose (GLU) were also evaluated by the blood biochemical analysis (Fig. 2(c)). There was no obvious change in these indicators, suggesting the non-toxicity of the NPs at the injected doses for one month. Recently, black TiO2 NPs have gained tremendous attention in tumor PTT due to their efficient conversion of light energy to heat and ROS [17, 18, 36-38]. In a study by Mou and co-workers, PEGylated black TiO2 NPs were synthesized by low temperature aluminium reduction and applied for dual image-guided PTT/PDT [37]. Interestingly, image-guided tumor therapy is at the fore-front of present-day strategies for cancer treatment [39, 40]. In order to exploit this strategy, Mou and co-workers further investigated the in vivo toxicity effects of pristine and PEGylated black TiO2 NPs to mice main organs (heart, spleen, kidney, lung, liver, and brain) and blood. They found that both the pristine and PEGylated NPs did not induce obvious toxicities in the mice main organs and blood after 20 days intravenous injection of 10 mg/kg NPs. Also, 2.5-10 mg/kg black TiO2 NPs containing Fe@γ-Fe2O3 were intravenously injected into healthy BALB/c mice in a study by Wang et al. [38]. They found that no obvious toxicity was induced by the NPs in comparison with the control (saline) group after 15 days. Saeed and co-researchers investigated the in vivo toxicity of black TiO2 (b-TiO2) NPs using the histological and hematological analysis following intravenous injection of the NPs into female Balb/c mice [17]. Typically, they harvested the organs (liver, heart, lungs, kidneys, and spleen) and also collected the blood of the mice 30 days post-injection of 500 µg/mL b-TiO2 NPs, followed by digestion with aqua regia at 90oC and quantification by ICP-OES. These researchers observed that the blood indices and parameters maintained normal levels (Fig. 3(a)). Also, no significant organ damage, lesion or inflammation occurred in comparison with mice control group (Fig. 3(b)). However, the b-TiO2 NPs accumulated in the livers, with low concentrations observed in the kidneys and hearts of the mice after 24 h post-injection of the NPs. These effects were also compared to those observed for Fe3O4 nanoflowers (Fe-NFs). More recently, the toxicity of Fe3O4-black-TiO2 nanocomposites (Fe-Ti NCs) was evaluated by intravenously injecting female nude mice with 200 µL (500 µg/mL) of the NCs [18]. The mice were sacrificed one month post-injection to collect their blood, hearts, kidneys, livers, spleens and lungs for hematological and histological analysis. They observed no toxic effect or behavioural change in the mice arising from the injection of the Fe-Ti NCs, while normal levels of blood indices and parameters were recorded. Taken together, these reports establish intravenous injection as a potential exposure route to TiO2 NPs, even at low doses.
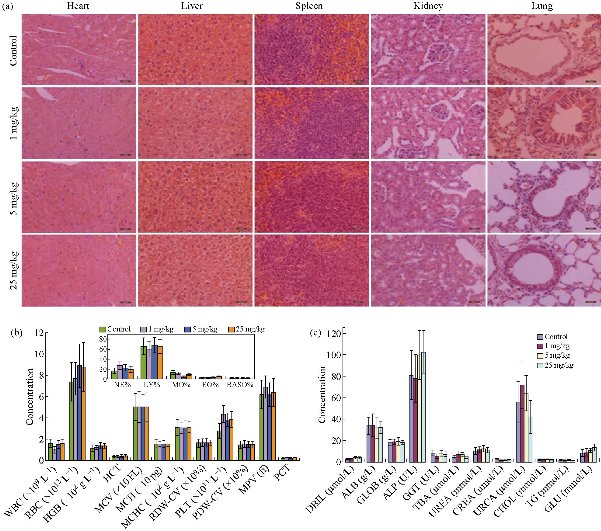
Fig. 2 (a) Histological analyses of mice main organs injected with saline or various doses of H-TiO2-PEG NPs (Scale bar = 20 μm). (b) Hematological and (c) blood biochemical analyses of the mice. Data are expressed as the mean ± standard (n = 3). Statistically significant differences were evaluated using the Student’s t-test (* p < 0.05, ** p < 0.01, ns > 0.05). Reproduced with permission [20].
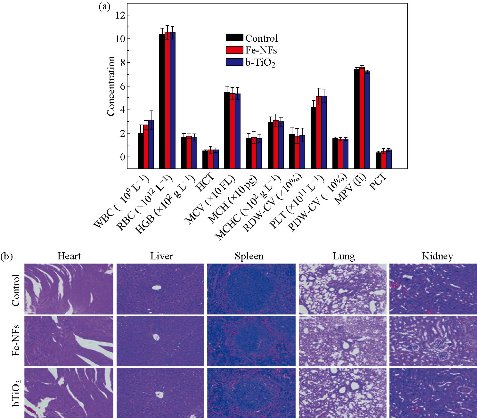
Fig. 3. (a) Blood analysis data of mice, 30 days post-injection of PBS, b-TiO2, and Fe-NFs. No significant changes in the blood parameters were observed. (b) Micrographs of H&E stained organs. No obvious changes in the mice organs were observed after the 30 days post-injection. Reproduced with permission [17].
Intraperitoneal injection
Though controversial, intraperitoneal injection is widely employed for the administration of chemotherapeutic drugs into humans for the treatment of certain cancer types such as ovarian cancer, where it has been recommended as a standard care method [41]. It particularly refers to the injection of an active ingredient into the body cavity (peritoneum) [42]. Intraperitoneal studies have been carried out to determine the toxicity levels of injected TiO2 NPs in humans or animal models [5]. To this end, Valentini and co-workers treated rats by intraperitoneally injecting TiO2 NPs with different doses (0.5-16 mg/kg) for the toxicity investigation of same [43]. Accumulation of Ti in the liver and kidney was observed by inductively coupled plasma atomic emission spectroscopy (ICP-AES). This accumulation induced physiological and morphological changes in the liver and kidney of the rats. In the liver, the hepatocytes located in the vicinity of the centrilobular veins were mostly affected. Furthermore, the toxicity of anatase-TiO2 NPs (5 nm) in mice after intraperitoneal injection (5-150 mg/kg body weight) for 14 days was investigated by Liu et al. [23]. They reported that the body weight coefficients of the lung and brain decreased, whereas those for the liver, kidney and spleen increased, while that for the heart showed little change. The TiO2 NPs caused damage to the liver, kidney, and myocardium, and also caused an impairment of the lipid balance and blood sugar in the mice. Therefore, they described the order of NP accumulation in the organs as liver > kidneys > spleen > lung > brain > heart. Younes and co-researchers reported an increase in blood platelet count after the intraperitoneal injection of TiO2 NPs (20 mg/kg) into adult Wistar rats for 20 days [44]. They reported that the injected NPs showed potential for inducing pathological changes in the liver of rats, following the increased accumulation of Ti in the liver, and the lung and brain of the rats (Fig. 4). This was determined by the increase in ratio of the aspartate aminotransferase (AST) to the alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) activities. Similarly, Guo and co-workers reported an increase in both the AST/ALT enzyme ratio and blood nitrogen urea [45]. This observation followed the intraperitoneal injection of high dose (500 mg/kg body weight) TiO2 NPs for 10 days, thus providing evidence for the toxicity of the NPs to the liver and renal system of Institute of Cancer Research (ICR) mice. In another study, TiO2 NPs (3.6 nm) were intraperitoneally injected (324, 648, 972, 1296, 1944 and 2592 mg/kg body weight) for 24 and 48 h, and then 7 and 14 days into mice [46]. The NPs were reported to have deposited in the liver, kidney, spleen and lung, and induced glomerular swelling, hepatocyte apoptosis and fibrosis in the mice. It then follows that intraperitoneal injection of TiO2 NPs at low and high doses have potentials for exposing animals to these NPs, with attendant toxicities.
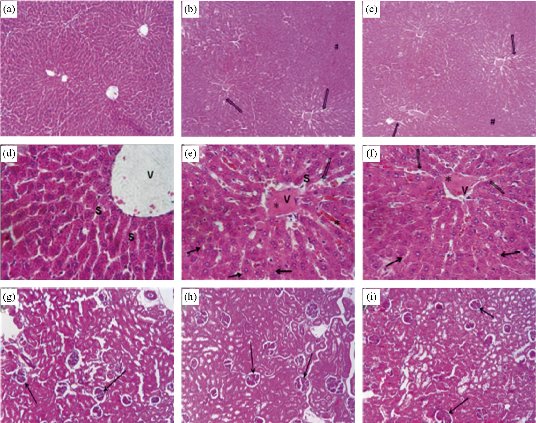
Fig. 4 Histopathology of the liver tissue (H&E stain) in male rats caused by intraperitoneal administration with TiO2 NPs. The rats were sacrificed (b, e) 1 day or (c, f) 14 days after the last injection and (a, d) control. a, b, c ×100 and d, e, f ×400. The open and closed arrows represent sinusoïdal dilatation and vacuoles, respectively; *congestion; #cellular space; vvein; and ssinusoid. Histology of kidneys of (g) control rats and treated rats sacrificed (h) 1 day or (i) 14 days after the last injection, ×100. The arrows represent the glomerulus. Reproduced with permission [44].
Subcutaneous injection and prenatal exposure
In a typical subcutaneous injection, the active ingredient is administered just below the skin, which is composed of mainly fatty tissues with little blood flow. As such, the injected active ingredient is slowly absorbed, sometimes up to or over 24 h [42]. Apart from TiO2 NPs, other substances that are injected subcutaneously include but not limited to epinephrine, insulin, and growth hormones [47]. The subcutaneous injection of TiO2 NPs to pregnant mice has been attributed to some pathological and functional impairments such as the olfactory bulb of the brain and reduction in sperm production [48]. To investigate this, Umezawa and co-workers subcutaneously injected 0.4 mg/body weight TiO2 NPs into pregnant mice on 6-15 days of gestation period [49]. It was observed that the NPs caused dysregulation of gene expression in the dopamine neuron region-related system and differential expression of genes which are associated with the striatum in the prenatal period. Similarly, TiO2 NPs were subcutaneously injected on gestation days 6, 9, 12, and 15 as reported in a study by Shimizu et al. [50]. Following a developmental investigation of male fetuses and pups using a contemporary DNA (cDNA) microarray analysis in combination with Medical Subjects Headings (MSH) terms information and gene ontology, changes in gene expression were observed. These changes were particularly recorded in the genes responsible for neurotransmitters and psychiatric diseases, brain development and apoptosis, and oxidative stress in the brain. Another study reported significant oxidative damage to the lipids and nucleic acids within the brain of new born mice [51]. Furthermore, during adulthood, depressive-like behaviors induced by stress during the gestation period were observed in the force-swimming and sucrose preference tests. It has also been reported that prenatal exposure to TiO2 NPs can affect the central nervous system, particularly the development of the central dopaminergic system in offspring [52]. To illustrate this, pregnant Slc:ICR mice were subcutaneously injected with 25-70 nm TiO2 NPs (100 μL at 1 mg/mL) on gestational days 6, 9, 12, 15, and 18. An investigation of the offsprings (newborn pups) revealed that the levels of monamines including dopamine, 3-methoxytyramine-hydrochloride, homovanillic acid, and 3,4-dihydroxyphenylacetic acid increased in the neostriatum and prefrontal cortex (Fig. 5(a)). Also, behavioral changes in the adult rats were observed at postnatal days 41 (Fig. 5(b)) and 43 (Fig. 5(c)). Put together, these reports reveal that various forms of toxicity such as oxidative stress and changes in gene expression can be traced to the subcutaneous injection as an exposure route to TiO2 NPs.
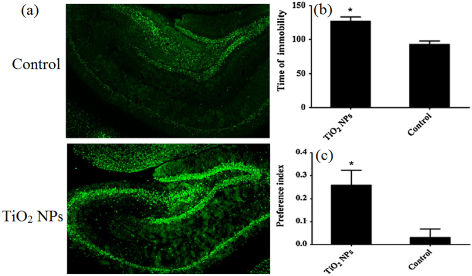
FIg. 5 (a) The effect of prenatal exposure to TiO2 NPs on the oxidative damage to nucleic acids in rat hippocampus. (b) The force-swimming test performed on postnatal days 41 and recorded as immobility behavior. (c) The sucrose preference test performed on postnatal day 43 and recorded as preference index. Preference index = (sucrose solution intake - tap water intake)/(sucrose solution intake + tap water intake) × 100. Reproduced with permission [52].
Dermal/skin exposure
The extensive utility of TiO2 NPs in sunscreens and other cosmetics has attracted widespread concern about the toxicity of these NPs as regards their potential to penetrate into the skin and enter the keratinocyte cell lines [7, 53]. However, under normal conditions, TiO2 NPs (an inorganic particle) may not have the potential for intact skin penetration owing to the strong protective effect of the stratum corneum (SC) [54]. The incapability of NPs to penetrate the skin has been demonstrated in some studies [55-57], while some other studies have shown otherwise [58-60]. In this regard, an in vitro study demonstrated that anatase-TiO2 (0–150 μg/ml) induced cytotoxicity in the HEL-30 mouse keratinocyte cell line [53]. It has also been demonstrated that TiO2 NPs could penetrate hairy skin when applied as an oil-in-water emulsion [58]. In a typical experiment, 5% of 20 nm TiO2 NPs was applied as an oil-in-water emulsion or aqueous suspension to human skin following the stripping method. It was observed that the NPs mainly from the oily dispersion penetrated through the hair pores or follicles of the human skin. In a related study, it was shown that short-term exposure to about 20 nm well-crystallized spherical-shaped TiO2 NPs could induce concentration-dependent biochemical impairment in the skin of Wister rats [61]. In the study (14-day acute exposure), 42 mg/kg of the TiO2 NPs was topically applied on the rat skin. Both renal and hepatic toxicity were observed in the treated rats attributable to the dermal exposure. It was therefore inferred that the NPs could have penetrated the rat skin through the hair follicles. Some other researchers opined that TiO2 NPs can penetrate via certain injuries such as sun burns and physical bruises on the skin [42]. On the other hand, Sagawa and co-workers demonstrated that non-coated and silica-coated TiO2 NPs did not penetrate the skin of mice, rats or human epidermis models [62]. The silica-coated and non-coated TiO2 NPs were suspended in Pentalan 408 and silicone oil, respectively, in a two-stage skin chemical carcinogenesis model. The animal models and their wide types were treated with 7,12-dimethylbenz[a]anthracene (DMBA) prior to TiO2 NPs treatments. No increase in the occurrence and abundance of skin tumors, particularly the carcinoma and squamous cell papilloma was observed in the animal models and their wide types after the DMBA initiation. This was attributed to lack of penetration of the skin by the TiO2 NPs. In a related study, mice were treated with DMBA and 12-o-tetradecanoylphorbol 13-acetate as initiator and positive control promoter, respectively, in a 20-week post initiation investigation of the two-stage skin chemical carcinogenesis [63]. Non-coated and industrial material-grade-coated TiO2 NPs (5, 10, and 20 mg/kg body weight) were applied to the skin of 7-week old female CD1 (ICR) mice. There were no observed changes in the body weight, survival rate, and general conditions of the mice models, suggesting that the NPs do no post-initiation potential for mice skin penetration and carcinogenesis. Furthermore, Crosera and co-workers applied 1.0 g/L TiO2 NPs in synthetic sweat solution on Franz cells using intact and needle-abraded human skin, and monitored the toxicity of the NPs for 24 h [64]. The cytotoxicity studies by the MTT and AlamarBlue® assays, and propidium iodide uptake revealed the absence of Ti in the receiving media after 24 h. However, 0.47 and 0.53 μg/cm2 of the TiO2 NPs were detected in the epidermal and damaged skin layer, respectively, after the 24-h exposure period. It was also reported in a related study that various sizes of TiO2 NPs (4, 10, 25, 60, and 90 nm) did not induce any form of toxicity as they could not penetrate through the stratum corneum [65]. This inference was made after the exposure of isolated porcine skin to the TiO2 NPs for 24 h. However, the 4 and 60 nm TiO2 NPs penetrated through the horny layer and were located in deep epidermis layer of pig ear after exposure for 30 days. Additionally, the various sizes of the TiO2 NPs penetrated the skin, reached different tissues, and induced diverse pathoogical damages in several major organs of mice, following a 60-day dermal exposure in hairless mice. It then follows that the toxicity potential of TiO2 NPs via dermal/skin exposure is hitherto controversial and require in depth studies.
Body implants
Recent advances in orthopaedic surgeries, especially endoprosthetics have been drammatically revolutionized by medical implants. However, autoimmune reactions have hampered the vast application of medical implants and cardiovascular stents [66]. In the light of this, researchers have over time employed Ti and its alloys, particularly nano-sized TiO2 as effective surface coatings for these body implants in order to mitigate the autoimmune reactions [67]. Furthermore, some researchers opined that in endo-prosthetic surgeries, safe tissue-recognition scaffolds are achievable with TiO2 NPs [68]. Following this, for the purpose of ameliorating tibia-tarsal or acetabulum hip joint fractures, Sul synthesized TiO2 nanotubes (15 and 700 nm thickness and length, respectively), and applied same for prosthetic articular surgery [69]. However, Ti-based implants undergo surface degradation (wear and corrosion) with consequent release of the corresponding metal ions and solid wear debris in the body, thereby leading to the popular peri-implant inflammatory reactions [70]. In a related study, TiO2 nanotubes were applied in enhancing strong bone adhesion in vivo, osteoblast adhesion in vitro, and bone mineralization [71]. It was shown in a pull-out testing after implantation of the nanotubes for four weeks that about a nine-fold enhancement in the strength of bone binding was attained compared with when TiO2 gritblasted surfaces were used. Additionally, new bone formation, greater phosphorus and calcium levels, and improved bone-implant contact area were observed on the surfaces of the nanotubes from the histological analysis. Under mechanical stress and/or impaired physiological conditions, there are indications that TiO2 NPs-based body implants can release some amounts of biologically related debris in both nanometer and micrometer ranges [25]. These debris are suggested to be associated with major systemic and inflammatory diseases [72]. In this regard, Wang and co-workers investigated the toxicity of anatase TiO2 NPs applied as medical implants on rat models [73]. They injected 0.2-20 mg/kg body weight of the NPs into the rats and observed that the major organs such as lung, liver, and heart were affected. In general, a maximum diameter of about 50 nm of the NPs moved across the synovial capillary wall, resulting in lymphocyte and plasma infiltration, fibroblast proliferation in the knee joint and synovial hypotrophy. There were also evidences of lipid peroxidation and oxidative stress in the exposed synovial fluid. Furthermore, in the alveolar macrophages and vascular endothelial cells, a brown deposit of particulates was observed, buttressing the toxic effect of the NPs. Put together, available literature reveal that irrespective of the size, body implants with TiO2 NPs provides an exposure route to these NPs.
Oral or gavage exposure
Nano-TiO2 has found application in food colorants, toothpaste, and nutritional supplements on a large scale. It is expected therefore, that the oral exposure to these NPs may occur via the consumption of such products [66]. The large scale utility of TiO2 NPs in food and other domestic products dates back to its approval by the United States Food and Drug Administration (FDA) in 1966, which allows up to 1% of the NPs in these products and food [74]. The gastrointestinal tract, an exchange/barrier system, is the most crucial entry route for NPs into the body [75]. The major absorption of NPs takes place via the epithelial villi and microvilli of the large and small intestines [25]. Recently, numerous efforts have been devoted to the development of effective carriers for the mitigation of oral exposure to nano- and microparticles [75-78]. Based on human intake and internal organ concentrations to account for long term accumulation, Heringa and co-researchers inferred that TiO2 NPs are potentially toxic to the liver and possibly the reproductive organs [74]. They attributed this toxicity to the consumption of the NPs via food, supplements and even toothpastes. In a study by Pele and co-researchers, 100 mg pharmaceutical/food grade TiO2 NPs were orally administered to human volunteers [9]. After 0.5 to 10 h of the ingestion, blood samples were collected and analysed for total Ti concentration by inductively coupled plasma mass spectrometry (ICP-MS) and for the presence of reflectant bodies (particles) by dark field microscopy. The results for the blood film analyses showed early absorption of the NPs (2 h) with a peak maximum at 6 h post-ingestion. The presence of these reflectant particles in the analysed blood samples roughly mirrored the levels of total Ti by ICP-MS, showing convincing evidence for the latter being a measure of the TiO2 NPs absorption. Furthermore, 250 mg/kg body weight of anatase TiO2 NPs (5 nm) were orally administered to mice for 30 days as reported in a study by Duan et al. [79]. They observed that whereas the treated mice lost weight, the relative weights of the liver, spleen, and kidney increased. The haemostasis of the immune system was severely affected, possibly due to the damage caused by the NPs on the spleen. Of course, the spleen is the largest immune organ in animals and as such, very crucial for immune response. In a related experiment, 2 mg/kg body weight of anatase TiO2 NPs (20-60 nm) were also orally administered to Sprague-Dawley rats per day for five consecutive days [80]. Following an ICP-MS analysis, a significant amount of Ti was observed in the spleen and the ovaries of rats which were exposed to high TiO2 NPs dose. The ICP-MS analysis was complemented with scanning electron microscopy (SEM) to establish the presence of particle agglomerates/aggregates in the spleen. Furthermore, orally administered TiO2 NPs have also been reported to cause lung cancer in rats [81]. The rats were administered with 40, 200, and 1000 mg/kg body weight of the NPs (33 and 160 nm) daily for seven consecutive days. It was also observed that the NPs induced micronuclei and DNA damage in bone-marrow cells and liver, apoptosis in the forestomach, and increased mitotic index in both the colon epithelia and forestomach. Contrary to the observed toxicity of orally administered TiO2 NPs on animal models, some researchers have reported no significant increases in Ti levels after treating some animal models with various doses of the NPs [82-84]. In a typical experiment, Sprague-Dawley rats were orally administered 5 mg/kg body weight of TiO2 NPs (40 nm) [83]. Again, following an ICP-MS analysis, no translocation of Ti into the urine or blood from the gastrointestinal tract was observed at 96 h post administration. Even after 4 days of administration, the liver, kidney and spleen obtained from the sacrificed rats showed no presence of Ti. Similarly, 1042 mg/kg body weight of TiO2 NPs (26 nm) were orally administered to Sprague-Dawley rats per day for 90 days as reported in a study by Cho et al. [85]. There were also no presence of Ti in the liver, kidney, brain tissues, and spleen of the rats as analyzed by ICP-MS, indicating low bioavailability of the NPs. Recently, Chen and co-researchers orally administered 2.5 mg/kg body weight of TiO2 NPs to mice per day for seven consecutive days, which was equivalent to the human dietary intake of TiO2 NPs [82]. No obvious toxicity was observed in the mice which were orally administered with the NPs, following investigations of their gut mitochondria and the development of colitis-like symptoms. Taken together, the biokinetic behaviour of TiO2 NPs is size dependent and there is a low systemic absorption of orally administered NPs with average sizes >100 nm [86]. This inference on the size-dependent toxicity of TiO2 NPs oral administration is supported by the report which showed that 25 nm anatase TiO2 NPs are more toxic than their 145 nm counterparts [87].
Inhalation (nasal) and intratracheal instillation exposures
The occupational and/or environmental exposures to NPs through inhalation may affect the respiratory system, resulting in increased risks of lung cancer, blockage of interalveolar areas, presence of inflammatory cells, and fibrosis [88]. These NPs can penetrate from the lungs to different other organs of the body via blood circulation and upregulate inflammatory proteins (MCP and MIP) and class I MHC genes by the Th2-mediated pathway [89]. Experimental evidence obtained from animal inhalation studies carried out using TiO2 NPs classified these NPs as occupational carcinogen by the National Institute for Occupational Safety and Health and as “possibly carcinogenic to humans” by the International Agency for Research on Cancer [25]. Undoubtedly, the exposure of humans to TiO2 NPs by inhalation can arise in work places during the handling of these NPs. Hitherto, no data is available to convincingly demonstrate the absorption of TiO2 NPs in humans through inhalation. However, it has been reported that factory workers in the production of TiO2 NPs may show adverse cytotoxicity responses via the inhalation of relatively high air-borne amounts of these NPs [88]. In this regard, Zheng and co-workers opined that the widespread utility of TiO2 NPs may increase the threat of combined exposure of these NPs with other environmental pollutants [90]. This was confirmed by their observation that in combination with bisphenol A, there was an increased movement of TiO2 NPs into exposed cells, resulting in a synergistic toxicity through oxidative stress, which induced micronuclei formation and DNA double-strand breaks. Muhlfield and co-researchers demonstrated that some fractions of TiO2 NPs (20 nm) were rapidly transported from the airway lumen to the interstitial connective tissue of male WKY/NCrl BR rats [91]. These rats were exposed to an aerosol containing 0.11 mg/m3 of the TiO2 NPs and each lung compartment was analyzed at 1 and 24 h post exposure by energy filtering transmission electron microscopy. It was also observed that the residence time of the NPs in each lung compartment of the respiratory system depended on the exposure time as the connective tissue and the capillary lumen were the preferential targets of the NPs at 1 and 24 h, respectively. In a related experiment, male Wister rats were exposed to 10 mg/m3 of TiO2 NPs in a 6-hourly inhalation study for five consecutive day [32]. Majority of the inhaled NPs were found to be deposited on the lungs, while some amounts translocated to the mediastinal lymph nodes. These deposited NPs were responsible for the observed macrophage activation and neutrophilic inflammation in the rat lungs. Over time, the lack of ultra-sensitive in vivo NP detection methods have made some researchers suggest that about 1% of NPs which are deposited in the lungs actually translocate to the blood and enter other vital organs [92, 93]. In the light of this, a nanoscale hyperspectral microscope was recently employed to spatially observe and determine the profile of TiO2 NPs after pulmonary deposition in adult female C57BL/6 mice [94]. The mice were exposed to 18 or 162 µg/kg body weight of the NPs via intratracheal instillation and were subsequently sacrificed after 24 h. The NPs were observed to have translocated to the heart and liver at low doses and to the blood at high NP doses. Moreover, an activation of complement factor 3 in blood, as well as inflammatory processes and complement cascade in the heart were also observed using ELISA analysis and global gene expression profiling. Furthermore, the biokinetics of 70 nm commercial and 48V-radiolabeled [48V] TiO2 NPs were investigated in female Wister-Kyoto rats in a study by Kreyling et al. [95]. They administered a single dose of 40-240 µg/kg body weight of the NPs via intratracheal instillation at retention time points ranging from 1 h to 28 d. The quantitative distribution of the NPs in all tissues and organs as determined with a high-sensitive radiotracer technique revealed the translocation of about 4% of the initial NP dose from the lung via the air-blood barrier at 1 h post exposure. These translocated NPs were mainly retained in the carcas, while about 0.3% remained after 28 d of the exposure. Similarly, Fischer 344 rats were exposed to 39.24 and 179.61 µg/kg body weight of TiO2 NPs by intratracheal instillation in a single and repeated dose, respectively, for 7 days [96]. Increases in bronchoalveolar lavage fluid neutrophils and heme oxygenase-1 were observed at 4, 8, and 24 h, and even after 7 d of the exposure. In summary, these reports point to inhalation and intratracheal instillation as a potential exposure routes to TiO2 NPs. In summary, studies point to various toxicity concerns of TiO2-based nanomaterials from the exposure routes discussed above, but with negligible reservations by some researchers. Notwithstanding, TiO2 NPs are extensively used in numerous aspects ranging from food industry to cancer treatment.
Environmental Impacts of Nano-TiO2
Owing to the vast utility of TiO2 NPs in food, industries, health care, and other consumer products, these NPs can after use get into the sewage system, subsequently entering into the environment biosolids applied to agricultural land, effluent discharged to surface waters, landfill solids, or incinerated wastes [10, 97]. Nano-sized TiO2 has over time demonstrated both positive and negative impacts on the environment. For instance, the photocatalytic ability of these NPs have been exploited in wastewater treatment and soil remediation for the photodegradation of various organic pollutants in water [98] and soil [99], respectively, and microbial films against pathogens [7]. More specifically, TiO2 NPs were recently applied for the alkaline medium-favoured photodegradation of methylene blue [100], and in earlier studies for the photodegradation of some pesticides such as methamidophos [101], atrazine [102], and chlorotoluron [103]. On the other hand, TiO2 NPs have been reported to induce various toxic effects on fish and other aquatic species [104], plants [105, 106], and soil biota [107]. Until now, reports on the ability of TiO2 NPs to mitigate air pollution or otherwise are quite vague [88, 108]. The following section will elaborate the influence of TiO2 NPs on environment.
Effects of TiO2 NPs on aquatic species
The wide industrial application of TiO2 NPs notwithstanding, little attention has been given to their environmental fates after disposal. Additionally, there is still no specific regulation for discarding nanomaterials into aquatic systems [109]. In aqueous solutions, TiO2 NPs tend to aggregate/agglomerate or interact with suspended organic matter, and then get deposited on the sediment [110]. However, about 52-86 μg/L of the TiO2 NPs remain suspended in the water column, mostly near sewages [111]. In either or both cases, TiO2 NPs discarded into aquatic systems will most likely be absorbed by the resident biota, with attendant negatives. Irrespective of all dilution and detection limit issues, TiO2 NPs from sunscreen lotions were detected in samples collected from the Old Danube Lake, Vienna, Austria in a study conducted by Gondikas et al. [104]. In some freshwater Teleostei, TiO2 NPs reduced the hepatic glutathione content and inhibited hepatic superoxide dismutase (SOD) and catalase (CAT) enzymes after subchronic exposure [112]. It has also been observed that TiO2 NPs are possibly taken up by freshwater Teleostei through their gills during respiration [113] and/or via diet through the intestine, and from these organs, the NPs may get to the blood stream [114]. In the blood, the TiO2 NPs may cause genotoxic damage (formation of micronuclei and nuclear abnormalities) to erythrocytes as it was observed in marine fish [115]. Carmo et al. reported the reduction of hemoglobin content and white and red blood cells in freshwater fish after exposure to TiO2 NPs [15]. They treated Juvenile fish with various amounts of TiO2 NPs (1-50 mg/L) for 48 h or 14 days as acute and subchronic exposures, respectively. It was also observed that the NPs demonstrated potential for increasing energy expenditure by affecting the immune system, thereby decreasing the ability of the fish to avoid predators and fight pathogens. In addition to the toxic effect of TiO2 NPs on blood cells, they may also be distributed to several organs through the blood stream, thereby modifying cellular metabolism and could possibly lead to some form of physiological imbalance of these affected organs [5, 15, 113]. Furthermore, exposure to TiO2 NPs may cause damages to liver tissues of aquatic species, such as the presence of disordered cells, apoptotic nuclei, and foci with lipid accumulation [114]. The liver, which is the main organ for xenobiotic metabolism in fish, may accumulate TiO2 NPs with a resultant redox imbalance [116]. Even at low concentrations, TiO2 NPs accumulated in other organs of fish such as the brain and muscle, thereby leading to an impairment in the metabolism of neurotransmitters [111, 116].
Effects of TiO2 NPs on algae and plants
Presently, there are limited information on the toxicity of TiO2 NPs to both algae and plants. However, Hou and co-researchers have provided a generalized illustration to enhance the understanding of the toxic effects of TiO2 NPs to algae and plants as shown in Fig. 6 [117]. Importantly, membrane and DNA damage, and cell growth inhibition bioassays remain the major parameters in determining the toxicity of TiO2 NPs to algae [118]. For instance, Wang and co-workers attributed the cell wall damage from the entrapment of algae to the toxic effect on the algae P. tricornutum [118]. This inference was made after the algae were exposed to TiO2 NPs at concentrations ≥ 20 mg/L for 5 days. They observed that aggregates of the NP entrapped the algae cells, thereby causing the algae cell wall damage even on the first day of the exposure. In a recent study, Sendra and co-researchers also attributed the cell membrane damage of four coastal microalgae (Amphidinium carterae, Chaetoceros gracilis, Nannochloropsis gaditana, and Pleurochrysis roscoffensis) to their exposure to TiO2 NPs [119]. This observation followed a 3-day exposure of the microalgae populations to various concentrations of TiO2 NPs in sunscreen products. They also observed that the differential sensitivity of the microalgae to the TiO2 NPs could cause an alteration in the phytoplankton dynamics, thereby provoking undesirable ecological effects such as the predominance of dinoflaggelates. As regards plants, Larue and co-workers investigated the fate of pristine TiO2 NPs and an aged paint leachate containing TiO2 NPs on lettuce leaves by foliar exposure [105]. They observed that the pristine NPs and those from the paint leachate were internalized in the lettuce leaves and in all leaf tissue types (Fig. 7). They also reported that no acute phytotoxicity was observed in the leaves and recorded low variations in phytochelatin and glutathione levels following the investigation of phytotoxicity markers. Szymanska et al. also investigated the response of a 5-week old plant (A. thaliana) to vitamin E, following exposure to TiO2 NPs [120]. They observed that an increase in the concentration of the NPs (100-1000 mg/L) resulted in corresponding increases in the biomass and chlorophyll contents of the plant. On the contrary, high concentrations of the NPs caused root growth and lipid peroxidation. Additionally, there was an alteration in the expression levels of tocopherol biosynthetic genes as the plant responded to the NPs. Recently, Dogaroglu et al. investigated the effect of TiO2 NPs on the antioxidant enzymes (SOD, CAT, glutathione, proline, and ascorbate peroxidase), chlorophyll content, and seed germination of Barley after 21 days of planting [121]. They reported that the seed germination was not affected by the application of the NPs. However, the chlorophyll contents decreased in comparison with the control group at TiO2 NPs concentration of 20 mg/kg. They also reported that the NPs caused an increase in the CAT and proline activities and a decrease in the SOD, glutathione, and ascorbate peroxidase activities of the Barley plant. In higher plants such as Nicotiana tabacum and Allium cepa, the toxic effects of TiO2 NPs have also been documented [106]. For instance, DNA damage was observed in Nicotiana tabacum, while lipid peroxidation, DNA damage, and growth inhibition were observed in Allium cepa after exposure to TiO2 NPs. Furthermore, increased level of MDA concentration at 4 mM TiO2 NPs was detected in Allium cepa, indicating that its DNA damage could also be attributed to lipid peroxidation.
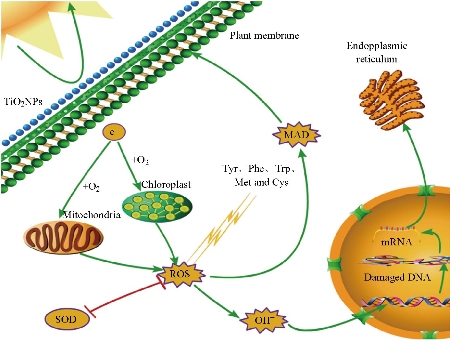
Fig. 6 Schematic illustration of the toxicity of TiO2 NPs to algae and plants. The UV irradiation of the NPs enables the generation of ROS and the subsequent damage to the DNA of the algae and plant cells. Reproduced with permission [117].
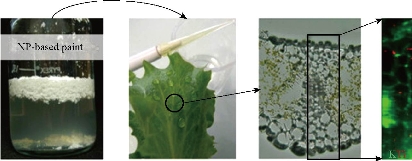
Fig. 7 Illustration of the phytotoxic effect of TiO2 NPs on the lettuce leaves showing their synchrotron based micro X-ray fluorescence map. The NPs from the paint leachate internalized in the leaves and in all types of the leaf tissues. Reproduced with permission [105].
Effects of TiO2 NPs on soil biota
Once released in the environment, TiO2 NPs could induce toxicity to non-target organisms in the environment, particularly taking the photocatalytic activity of these NPs into account [122, 123]. With respect to soil organisms, the reported apical (survival and reproduction) effects of TiO2 NPs show a broad EC50 span ranging both below and above 1000 mg/kg of TiO2 [7, 124]. Unfortunately, there is no much information on the widespread gene-expression responses in soil organisms, which may have provided an explanation to the observed apical toxicity and thus enable a better understanding of what actually happens following biological exposure to these NPs [7]. Although an in vitro study showed no obvious toxicological effect of TiO2 NPs on lysosomal function, plasma membrane integrity, and cell mitochondrial activity [125], these NPs have been reported to induce DNA damage [107] and oxidative stress [126] at the sub-organismal level. The recent study by Gomez and others [7] on the toxicity of photoactivated TiO2 NPs to Enchytraeus crypticus showed that the photoactivity of the NPs enhanced their toxic effects. Particularly, lysosome damage, negative effect on reproductive organs, and activation of oxidative stress transcription were observed. Also, the response of the microbial community of soil for agricultural purpose to a 90-day exposure to TiO2 NPs was investigated in a study by Simonin et al. [14]. In order to ascertain their soil function impacts, the researchers monitored the nitrogen cycle and also measured the nitrification and denitrification activities of enzymes. They further quantified specific representative genes including nirS and nirK for denitrifiers, and amoA for the ammonia oxidizers. Furthermore, an investigation into the diversity shifts in archaea, bacteria and their corresponding ammonia-oxidizing clades was conducted. These researchers observed strong negative effects attributed to the TiO2 NPs on the abundances of ammonia oxidizers and nitrifying enzyme activities. The TiO2 NPs also induced a large bacterial community modification and cascading negative impacts on the activities of denitrification enzymes. Their observations therefore suggest for more detailed research into the manner in which TiO2 NPs modify soil health and the ecosystem function at large. In summary, notwithstanding the fact that TiO2 NPs have been extensively applied for advantageous environmental cleanup such as in soil remediation and water treatment, numerous studies have showcased the toxicity of such NPs to aquatic life, soil biota, algae and plants. However, few researchers argue that TiO2 NPs are not toxic, especially to soil biota. These few studies were mainly conducted in vitro, which necessitates complementary in vivo studies to establish or debunk such safety claims.
The Mechanism of TiO2-Induced Toxicity
Nano-sized TiO2-induced toxicity follows various mechanistic pathways such as cellular uptake, oxidative stress, neurotoxicity, genotoxicity, and immunotoxicity. In the following sub-sections, these specific mechanistic pathways will be elaborated in detail. Notwithstanding, a generalized potential mechanism for TiO2-induced toxicity is summarized in Fig. 8.

Fig. 8 The potential of TiO2-induced toxicity mechanism. Triggered by UV irradiation, the TiO2 NPs can reach the cell mitochondrion and also cause damage the DNA. Reproduced with permission [117].
Cellular uptake of TiO2 NPs
Nano-sized TiO2 may cross cell membranes by processes including endocytosis, diffusion (adhesion interactions), and binding to cellular receptors [15]. In an experiment investigating the chemotherapeutic effect of TiO2-PEG-DOX NPs on breast cancer (MDA-MB-231-GFP-fLuc) cells, Balb/c mice were exposed to various amounts of TiO2 for 15 days [127]. Irrespective of the fact that an examination of the in vivo toxicity of the NPs showed no obvious body weight loss or changes in the major organs (histological analysis) of the treated mice, Fig. 9 shows the cellular internalization of the NPs which could be particularly observed in Fig. 9(e)-(h) and (q)-(x), thus demonstrating cellular uptake as a mechanistic pathway for the toxicity of TiO2 NPs. In a study demonstrating the MRI-guided photothermal therapeutic efficacy of a black TiO2-based nanoprobe, cellular uptake of the NPs by pancreatic cancer stem cells was examined at 2 and 4 h [128]. For this purpose, the nanoprobe (designated as bTiO2-Gd-CD133mAb) was prepared by conjugating black TiO2 NPs with an MRI contrast agent (DOTA-Gd) and CD133 monoclonal antibodies (CD133mAbs). The ability of the nanoprobe to target CD133 which is highly expressed on pancreatic cancer stem cells (S, red fluorescence in Fig. 10) was determined by synchrotron radiation hard X-ray fluorescence microscopy. Although low cytotoxicity of the TiO2 NPs was reported in the study, cellular uptake provides a mechanistic pathway for potential interaction of cells with the NPs which might show toxicity to such cells at later dates. Cellular uptake as a mechanistic pathway for the induced toxicity of TiO2 NPs was also observed in a study by Sentilkumar and Rajendran [129]. In a cellular uptake investigation using MCF-7 and MDA-MB-231 cells, the researchers observed that the NPs significantly altered the morphology of these cells. This was complemented by the marked decrease in cell viabilities of both cell lines treated with the TiO2 NPs at Ti concentration of 1.0 µM in comparison with the untreated cells as control. Additionally, a similar report showed strong Ti signal intensity in a cellular uptake investigation of TiO2 NPs by MCF-7 cells [35]. The Ti was reported to have accumulated mainly in the cytoplasm of the cells, while some amounts of the element were visualized around the nucleus of the cells by synchrotron X-ray fluorescence microscopy after 2 h of incubation. These findings further suggest cellular uptake as a mechanism for the potential toxicity of TiO2 NPs.

Fig. 9 Confocal microscopic images for the internalization of TiO2–PEG–DOX NPs in the MDA-MB-231-GFP-fLuc breast cancer cells. (a)-(d) Control group. (e)-(h) TiO2–PEG NP treatment of cells. (i)-(l) and (m)-(p) Cells incubated with DOX for 2 and 4 h, respectively. (q)-(t) and (u)-(x) Cells incubated with TiO2–PEG–DOX NPs for 2 and 4 h, respectively (scale bar = 50 µm, 40 ×). Reproduced with permission [127].
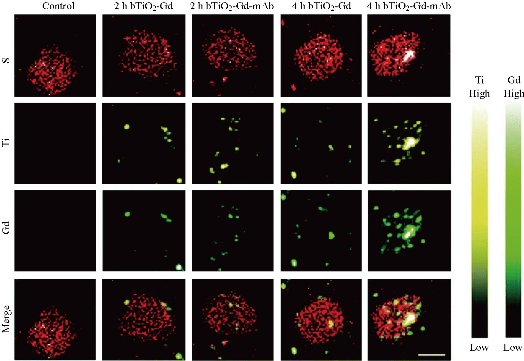
Fig. 10 X-ray fluorescence microscopic images of CD133 highly expressed pancreatic cancer stem cells incubated with the bTiO2-Gd and bTiO2-Gd-CD133mAb nanoprobes for 2 and 4 h. Biogenic S element in cells is shown as red-bright color, while Ti and Gd elements of nanoprobes are shown as yellow-bright and green-bright colors, respectively. Scale bar = 10 µm. Reproduced with permission [128].
Oxidative stress induced by TiO2 NPs
Oxidative stress caused by TiO2 NPs following the generation of ROS has been described by some researchers as a possible toxicity mechanism for TiO2 NPs [130, 131]. In this regard, a recent study by Deng et al. attributed ROS generation to the physiological effects observed in Phaeodactylum tricornutum (P. tricornutum) which included changes in soluble sugar, chlorophyl a, and malondialdehyde (MDA) contents, and peroxidase (POD) and SOD activities [130]. Similarly, Li and co-researchers attributed the growth inhibitions and lipid oxidations of Karenia brevis (K. brevis) and Skeletonema costatum (S. costatum) by TiO2 NPs (average size of 5-10 nm) to ROS-mediated oxidative stress [131]. It was observed that algae chloroplast was the specific site for the ROS generation and accumulation, following the addition of inhibitors of various electron transfer chains. Additionally, the EC50 values of the TiO2 NPs to K. brevis and S. costatum were 10.69 and 7.37 mg/L, respectively, after 72 h of exposure. Obvious effects such as increases in MDA and changes in SOD and POD activities also resulted from the TiO2 NPs exposure. Furthermore, the light-induced increase in toxicity and tocochromanol content in Arabidopsis thaliana (A. thaliana) following exposure to TiO2 NPs (100-1000 mg/L) has been attributed to the photoactivation of the NPs and enhancement of ROS generation [120]. It was also observed that high concentrations of the NPs caused lipid peroxidation. Moreover, there were either down- or up-regulation of the expression levels of tocopherol biosynthetic genes in response to the TiO2 NPs. In another study, oxidative stress was also reported in newborn pups following the prenatal exposure of adult Sprague–Dawley rats to 500 µL (1 µg/µL) TiO2 NPs by subcutaneous injection [51]. The NPs were injected on gestational days 6, 9, 12, 15, and 18, while the pups were examined 2 days after birth. The observed toxicity in the pup organs was attributed to the oxidative stress induced by the TiO2 NPs. Summarily, oxidative stress induced by TiO2 NPs has been demonstrated in these reports as a potential mechanism for TiO2 NPs toxicity.
Neurotoxicity of TiO2 NPs
Nano-sized TiO2 has been described as having the capacity of crossing the blood-brain barrier and as such with potential for nuerotoxic effect, as reported in a study by Chen et al. [132]. This was illustrated by these researchers in the time-dependent accumulation of TiO2 NPs in the brain of Zebrafish (Danio rerio), following a long-term (6 months) exposure of the fish to 7 mg/L of the NPs. This was the first report on the neurotoxicity of TiO2 NPs on Zebrafish, after long-term exposure. Also, the brain of newborn Sprague–Dawley rat pups were affected by prenatal exposure of adult rats to 1 µg/µL (500 µL) TiO2 NPs at different gestational days [51]. The brain tissues of male pups were examined on postnatal day 2. It was observed that the NPs crossed the blood-brain barrier and induced damages to the lipids and nucleic acids of the pup brains. In a recent investigation, the short-term co-exposure (21 days) of Zebrafish to 100 µg/L TiO2 NPs and bisphenol A (2-200 µg/L) was conducted to ascertain the neurotoxicity of the TiO2 NPs in conjuction with another compound. The TiO2 NPs sorbed the bisphenol A and the resulting NPs were reported to have also crossed the blood-brain barrier [116]. This was explained by the accumulation of about 120-150 µg/g of the NPs in the Zebrafish brain. The study therefore highlighted that the observed neurotoxicity was synergistically contributed by both components of the NPs. Additionally, TiO2 NPs were reported to have crossed the blood-brain barrier and accumulated in the brain of a Neotropical detritivorous fish (Prochilodus lineatus) and posed neurotoxic effects [15]. This was inferred following the decrease in the enzyme acetylchlorinesterase activity after an acute exposure (2 days) of 1-50 mg/L TiO2 NPs. Another recent study also reported the neurotoxic effect of TiO2 NPs in a dose-dependent manner [133]. The study evaluated the accumulation of the NPs in mice brain after intraperitoneal administration. The histological changes in the mice brain with the control group (Fig. 11(a)) and TiO2 NPs groups (Fig. 11(b)-(d)) show the cracked and ruptured nerve cells (Fig. 11(c)) and the infiltration of the inflammatory cells in the mice brain (Fig. 11(d)), after treatment with 100 and 150 mg/kg body weight TiO2 NPs, respectively. Moreover, the activities of some enzymes such as the inducible nitric oxide synthases, acetylchlorinesterase, and constitutive nitric oxide synthases, and the levels of glutamic acid and nitrous oxide in the mice brain were altered after the exposure to the NPs. These findings suggest neurotoxicity as a potential toxicity mechanism for TiO2 NPs.
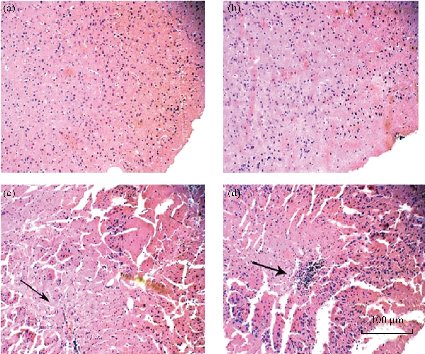
Fig. 11 Pathological changes in mice brain tissue exposed to TiO2 NPs. The mice were treated with the TiO2 NPs or saline solution once a day for 14 days, through intraperitoneal injection. (a) Control; (b) 50, (c) 100, and (d) 150 mg/kg body weight of the NPs. The TiO2 NPs caused the cracking and rupturing of nerve cells and the inflammation of brain cells of the mice. Reproduced with permission [133].
Genotoxicity of TiO2 NPs
In order to evaluate the in vivo genotoxicity of TiO2 NPs, parenteral administration was reported to be capable of achieving sufficient systemic exposure of the NPs after oral uptake, due to the low absorption of TiO2 particles [86]. In a different study, the genotoxicity of anatase TiO2 NPs was investigated by assessing the micronuclei in reticulocytes and the mutation frequency in the Pig-A gene in fringe blood cells [134]. For this purpose, 0.5-50 mg/kg body weight of the NPs (average size of 10 nm) were intravenously injected into B6C3F1 male mice for 3 consecutive days. It was observed that even though the NPs induced cytotoxicity in the mice bone marrow, they did not cause obvious direct genotoxicity.
Furthermore, Dobrzynska and co-researchers exposed Wistar rats to a single dose of 5 mg/kg body weight anatase/rutile TiO2 NPs (average size of 21 nm) by intravenous injection [135]. No obvious genotoxicity was detected in the red and white blood cells of the bone marrow after 24 h, and 1 and 4 weeks exposure of the rats to the NPs. However, about a 3-fold increase in the micronucleated cells was observed after 1 h of exposure, following polychromatic srythrocytes staining with May-Grunwald-Giesma reagents. On the contrary, male mice were exposed to 500-2000 mg/kg body weight of anatase/rutile TiO2 NPs (average size of 45 nm) by interperitoneal administration for 5 consecutive days [136]. The potential genotoxicity induced by the NPs was assessed by monitoring the appearance of breaks in DNA strands when the bone marrow, liver and brain cells were subjected to the comet assay, and counting the micronuclei frequency in bone marrow polychromated erythrocytes. A statistically significant dose-dependent increase in DNA strand breaks and micronuclei frequency was observed after 24 days of the last TiO2 NPs exposure. In another study, Driscoll and others reported an increase in the mutation frequency of hypoxanthine phosphoribosyltransferase (HPRT) in alveolar type II cells after 15 months of rat exposure to 100 mg/kg body weight of anatase TiO2 NPs (average size of 18 nm) [137]. Additionally, Ghosh and co-researchers reported that TiO2 NPs could cause genotoxic effects when they carried out a comparative cytotoxicity and genotoxicity study between TiO2 NPs and bulk TiO2 [106]. They observed that the TiO2 NPs induced genotoxicity at a dose of 0.25 mM, while the bulk TiO2 induced genotoxicity at concentrations of 1.25 mM and above to human lymphocytes. Recently, Gomez and co-researchers reported that mice exposure to 1 mg/L TiO2 NPs affected gene transcription and translation [7]. They utilized three different TiO2 NPs (denoted as NM103, NM104, and NM105), which varied in their surface modifications, and assessed their toxicities against bulk TiO2. Whereas NM105 was not surface modified, both NM103 and NM104 were coated with a thin shell of Al2O3 and separately treated with dimethicone (to enhance hydrophobicity) and glycerin (to enhance hydrophilicity), respectively. They observed that the NPs induced gene alterations as there were more differentially expressed genes (DEGs) in the NPs than in the bulk TiO2, even without UV irradiation (Fig. 12). Furthermore, another recent investigation on the effect of high-dose TiO2 NPs revealed both developmental and genetic toxicity of the NPs to mice embryo [133]. Moreover, the study also reported disorders in the expression of protective genes in the mice liver after interperitoneal administration of the NPs. In another study, membrane damage and genotoxicity caused by TiO2 NPs was reported in four coastal marine microalgae namely: Amphidinium carterae (Dinophyceae), Chaetoceros gracilis (Bacillariophyceae), Nannochloropsis gaditana (Eustigmatophyceae), and Pleurochrysis roscoffensis (Primnesiophycae) [119]. The study was conducted by exposing the microalgae populations to three commercial sunscreen products (with variable TiO2 concentrations) for 3 days in the presence of UV radiation. Taking cognizance of other organic compounds in the sunscreens which may also affect the toxic effects of the products, the genotoxicity was mainly attributed to the UV-activated TiO2 NPs components of the sunscreens. In summary, these findings generally indicate that the possibility for TiO2 NPs to induce genotoxic effects to various organisms cannot be overruled at high NP doses.

Fig. 12 (a) Number of DEGs (control/treatment ratios), and (b) Venn diagram representation of the DEGs affected by exposure to 1 mg/L of the TiO2 materials: bulk, NM103, NM104, and NM105, without or with UV irradiation in ISO water for 5 days. Down/up represents down- or up-regulated transcripts. The NPs induced gene alterations evidenced by the number of more DEGs in the NPs than in the bulk TiO2. Reproduced with permission [7].
Immunotoxicity of TiO2 NPs
Nano-sized TiO2 has recently been reported as potentially immunotoxic to a fish (Prochilodus lineatus) as it altered the immune system and increased the energy expenditure, thereby reducing the ability of the fish to avoid predators and fight pathogens [15]. In the study, a subchronic exposure (14 days) of the fish to 1-50 mg/L TiO2 NPs caused a decrease in red blood cells, white blood cells, and lymphocytes, and an increase in the mean cell volume and hemoglobin. In another study, increased tissue burdens of TiO2-bisphenol A NPs have been observed after a short-term exposure of male and female Zebrafish to these NPs [116]. The study reported that the plasma concentrations of testosterone, estradiol, and follicle-stimulating hormone decreased after the 21-day co-exposure of male Zebrafish to the NPs. For the female zebrafish, the co-exposure was reported to have caused decreases in the concentrations of the follicle-stimulating hormone and luteinizing hormone. The local immune function of rats exposed to 0.5-50 mg/kg body weight of TiO2 NPs (average size of 5 nm) by intratracheal instillation was reported in a study by Liu et al. [138]. High-dose exposure of the rats to the NPs caused a decrease in the phagocytic ability of the pulmonary alveolar macrophages, while low-dose exposure increased this ability. Also, the NPs decreased chemotactic ability and increased the secretion of tumor necrosis factor-alpha (TNF-α) of the macrophages, thus suggesting that the NPs could disrupt macrophage functions associated with pulmonary specific and non-specific immunities. Nano-sized TiO2 was also detected in the immune cells of rats which were orally exposed to the NPs for one week at human relevant levels [139]. Particularly, the Peyer's patches of the rat immune cells showed increased frequency of dendritic cells, while a decrease in regulatory T cells which are involved in reducing inflammatory responses were observed following the oral exposure. Furthermore, a decrease in the secretion of Thelper (Th)-1 IFN-γ and a sharp increase in splenic Th1/Th17 inflammatory responses were observed following the stimulation of the immune cells isolated from the Peyer's patches of the rats. Systemic immune effects have also been observed after the exposure of Sprague-Dawley rats to TiO2 NPs [140]. The rats were exposed twice a week for three consecutive weeks to the NPs at doses ranging from 0.5-32 mg/kg body weight. The histopathological immune organs obtained from the exposed rats showed slight spleen congestion and brown particulate depositions in the axillary and cervical lymph nodes. Additionally, the immune function response of the rats was characterized by an increase in the T and B cells proliferation following mitogen stimulation. An enhancement in the cell killing activity of the spleen, coupled with an increase in the number of B cells in the blood was also observed in the exposed rats. Taken together, available literature reveal that immonotoxicity is potential mechanism for the toxicity of TiO2 NPs.
Conclusions and Future Perspectives
Since the early discovery of ultraviolet radiation-mediated water splitting on the surface of TiO2, research on the diverse applications of TiO2 NPs has been on the rise. Complementarily, the FDA approval of TiO2 as a food additive has increased the worldwide production and consequent availability of TiO2 and its NPs for various research purposes. Presently, TiO2 NPs have numerous gainful applications due to their exceptional properties, but the toxicity of these NPs to date is not fully understood, which is common for most other NPs. Interestingly, some researchers argue that TiO2 NPs present obvious toxicity to cells, major animal organs, and the environment at large, while some researchers argue otherwise. Nevertheless, it has been established that intravenous, intraperitoneal, subcutaneous, and intra-articular injections, prenatal, dermal, body implants, oral, inhalation, intratracheal instillation, abdominal/intra-abdominal, and intragastric administration account for the numerous exposure routes of animals to TiO2 NPs. Apart from animals, other organisms such as soil biota and aquatic species, and various plant species have been negatively affected by TiO2 NPs. Additionally, various mechanisms have been put forward to describe the toxicity of TiO2 NPs. These include oxidative stress, cellular uptake, neurotoxicity, genotoxicity, and immunotoxicity. There are numerous ways in which the toxicity of TiO2 NPs can be ameliorated. We observed that some researchers reported a dose-dependent toxicity of TiO2 NPs and we therefore opine that adequate functionalization of these NPs can extensively enhance their efficacies and reduce the need for excessive administration of the NPs to animals or their use in various environmental aspects. In medical implants for instance, it is necessary to develop highly crystalline TiO2 NPs with improved stability in order to reduce the toxic effects of the NPs. Furthermore, proper encapsulation of these NPs into other nanomaterial carriers to form slow release systems for the photodegradation of organic pollutants can effectively mitigate their environmental toxicities by taking advantage of the benefits of the slow release technology. This encapsulation strategy can also be extended to the application of TiO2 NPs for photothermal and photodynamic cancer therapies, wherein the nanocarriers can release the NPs at the tumor microenvironment. We therefore envisage that this work, which showcases the recent toxicity assessments of TiO2 NPs will also serve as a useful baseline information for an adequate future conventional classification of TiO2 NPs toxicity.
Acknowledgements
The authors acknowledge the support of National Key R&D Program of China (2018YFC0910601, and 2019YFA04000803), National Natural Science Foundation of China (31971292, U1432114, 81650410654, 8161101589), the Hundred Talents Program of Chinese Academy of Sciences (2010-735), Key Breakthrough Program of Chinese Academy of Sciences (KGZD-EW-T06), Special Program for Applied Research on Super Computation of the NSFC-Guangdong Joint Fund (the second phase to Aiguo Wu, U1501501), and the Science & Technology Bureau of Ningbo City (2015B11002). Ozioma Udochukwu Akakuru also appreciates the Chinese Academy of Sciences (CAS) and The World Academy of Sciences (TWAS) for the award of the CAS-TWAS President's Fellowship (2017A8017422001) for PhD studies.
Competing Interests
The authors declare no competing interest.
References
Copyright© Ozioma Udochukwu Akakuru, Muhammad Zubair Iqbal, Chuang Liu, Zihou Li, Yang Gao, Chen Xu, Elvis Ikechukwu Nosike, Gohar Ijaz Dar, Fang Yang, and Aiguo Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.