Research Article
Cyclic Voltammetry Study for MnO2 Nanoparticles Modified Carbon Paste Electrode
Emad Salaam Abood 1*, Mothana Salih Mashkoor 2, Amer Mousa Jouda 2
1 Department of Medical physics, Hilla University College, Babylon, Iraq.
2 Department of Chemistry, Faculty of Science, University of Kufa, An-Najaf, Iraq.
* Corresponding author. E-mail: amadiraq45@gmail.com
Received: Sep. 4, 2019; Accepted: Nov. 29, 2019; Published: Nov. 29, 2019
Citation: Emad Salaam Abood, Mothana Salih Mashkoor, and Amer Mousa Jouda, Cyclic Voltammetry study for MnO2 Nanoparticles Modified Carbon Paste Electrode. Nano Biomed. Eng., 2019, 11(4): 368-374.
DOI: 10.5101/nbe.v11i4. p368-374.
Abstract
An electrical method was prepared for the measurement of Mn with trace amount in drugs and for the assessment of a sensitive method in comparison with spectrometric and atomic analyzer by a new carbon paste electrode modified with MnO2 nanoparticles. The method was used to study the electro oxidation of manganese ions in solution by cyclic voltammetry (CV) method. The modified electrode displayed strong resolving function for the overlapping voltammetric response of Mn-ion into one well-defined peak. The potential difference between Epa and Epc was > 200 mV; this range referred to the quise-reversible mechanism. The kinetic of electrode was studied at the temperature range from 15 - 35 °C; the data of voltamograms showed the increase of temperature caused increase of negative shift, which suggested the diffusion electron transferred in the redox process of Mn-ion oxidation. Diffusion coefficient was calculated from the Randles-Sevcik equation and was equal to 1 × 10-7; the rate constant K was equal to 5.3 × 10-5; the peak current of Mn-ion increased linearly with its concentration at the range of 0.5 - 4.5 ppm.
Keywords: MnO2 nanoparticles; Oxidation-reduction; Carbon paste electrodes
Introduction
Drug analysis plays an important role in drug quality control, and has great impact on public health. Therefore, a simple, sensitive and accurate method for the determination of active ingredient is a crucial step [1].
Magnesium
Many supplement manufacturers use a less expensive, inorganic form of this mineral known as magnesium oxide. For example, a manufacturer may claim that their multivitamin contains an impressive 200 or 400 mg of magnesium per serving, which may at first appear substantially higher than that of a neighboring product. However, if it is in the form of magnesium oxide, studies have shown that its absorption rate may be as low as 4%. Alternatively, an organically chelated or food state form of magnesium may yield only 50 to 100 mg per serving, but its bio-availability has been shown to be substantially higher than that of oxide in multiple studies. Simply put, you'll get more out of it [2, 3]. Magnesium dioxide is used as a supplement to maintain adequate magnesium in the body. Magnesium oxide is also used as an antacid to treat indigestion, or as a laxative to relieve occasional constipation. Magnesium is a mineral that is important for normal bone structure in the body. People get magnesium from their diet, but sometimes magnesium supplements are needed if magnesium levels are too low. Dietary intake of magnesium may be low, particularly among women. Magnesium deficiency is also not uncommon among African Americans and the elderly. Low magnesium levels in the body have been linked to diseases such as osteoporosis, high blood pressure, clogged arteries, hereditary heart disease, diabetes, and stroke.
Constipation
Taking magnesium by mouth is helpful as a laxative for constipation and to prepare the bowel for medical procedures.
Indigestion (dyspepsia)
Taking magnesium by mouth as an antacid reduces symptoms of heartburn and indigestion. Various magnesium compounds can be used, but magnesium hydroxide seems to work the fastest.
Seizures in women with pre-eclampsia
Administering magnesium intravenously (by IV) or as a shot is considered the treatment of choice for eclampsia. Administering magnesium reduces the risk of seizures in women with this condition.
Low levels of magnesium in the blood (hypomagnesemia)
Taking magnesium is helpful for treating and preventing magnesium deficiency. Magnesium deficiency usually occurs when people have liver disorders, heart failure, vomiting or diarrhea, kidney dysfunction, and other conditions.
A pregnancy complication marked by high blood pressure and protein in the urine (pre-eclampsia)
Administering magnesium intravenously (by IV) or as a shot is considered the treatment of choice for preventing seizures in women with pre-eclampsia [4]. Thus, a quantitative determination of manganese concentration is significant for developing immune physiological and pharmacological research and life sciences. There are some methods applied to the determination of manganese, such as flow injection, fluorimetry [5] and spectrophotometry method [6]. As an electro active device, it can also be studied via electrochemical techniques. Some reports showed the electrochemical response of manganese on manganese dioxide nanoparticle carbon paste electrode [7-8]. MnO2 is a promising electrode material for electrochemical capacitors because of its the relatively low cost, excellent electrochemical performance, environmentally friendly character in comparison with the ruthenium oxides or other transition metal oxides [9-10]. Many kinds of nanoparticles, including metal nanoparticles, oxide nanoparticles, semiconductor nanoparticles and composite nanoparticles have been widely used in electrochemical sensors and bio sensors [11-14]. Copper is electro active component that can be determined electrochemically [15].
Working electrode
A calomel electrode and a platinum (Pt) wire were employed as the reference electrode and counter electrode, respectively, and all potentials were reported with respect to the former. A metrohm pH/ ion meter was used for pH measurements. All solution was freshly prepared with doubly-distilled water (conductivity of water was 0.055 μS/cm). All reagents were of analytical grade from Merck. Graphite powder and paraffin oil were both from Merck, used as received. High dilution acid-base was in the pH range of 4.0 - 8.0 [16-17].
MnO2 nanoparticles electrode
Manganese oxides (MnO2) are the most potential one because of their low cost, natural abundance, high theoretical capacity (w1370 F g1), non-toxicity and wide operating potential window in mild electrolyte. However, bulk MnO2 suffers from low electronic conductivity, low ionic diffusion constant and structural susceptibility. This stimulates an extensive interest in developing MnO2-based composites with conductive materials, such as carbon nanotubes, graphene, carbon nanofibers and conducting polymers, etc., in order to overcome their drawbacks [18-19]. We worked on making MnO2 nanoparticles to improve their properties.
Preparation of MnO2 nanoparticle by hydro-thermal method
MnO2 nanoparticles were synthesized by hydrothermal method. Typically, 8 g of MnCl2 was dissolved in 150 mL of distilled water, and then a solution containing 8 g of NaOH was slowly added with stirring. After 8 h, a dark grey precipitate was obtained. The resulting precipitate was washed with distilled water and dissolved again in water to get solution, which was mixed with 50 mL of poly vinyl polyline (PVP) solution (13 g of PVP dissolved in water) solution during a continuous stirring to get a homogeneous solution. The homogeneous solution was transferred into autoclave and heated at 160 °C for 8 h. Then, MnO2 powder was obtained and dried at 100 °C after it had been washed with distilled water. The morphology of the synthesized MnO2 nanoparticle sample was studied by scanning electron microscopy (SEM) by using SEM Inspect S50 (Fig. 1).
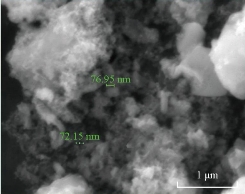
Fig. 1 Scanning electron microscopy (SEM) image of MnO2 nanoparticles.
Experimental
Apparatus and chemicals
The electrochemical measurements were performed with an auto lab potentiostat (Digi-ivy 2113 Texas, USA) controlled by the general-purpose electrochemical system software. The MnO2 nanoparticle carbon paste electrode was used. The MnO2 modified carbon paste electrodes were prepared by mixing 0.3 g of MnO2 nanoparticles, 1 g graphite powder, and 0.8 mL of paraffin oil with a mortar and pestle, until a uniform paste was obtained. The paste was packed into the end of a glass tube (diameter of 0.44 mm and 10 cm long). The electrical contact was provided by inserting a copper wire into the carbon paste. Prior to the experiment, the surface of the carbon paste was polished with fine paper. The unmodified carbon paste electrode was prepared in the same way without adding MnO2 nanoparticles to the paste.
Results and Discussion
Cyclic voltammetry study of manganese
The SEM image shows the agglomerated particles with an average size of 72 and 76 nm (Fig. 1). MnO2 nanoparticles have a large number of potential applications in the field of sensors, catalysis, pharmaceutical industries, piezoelectric crystals and electrodes of fuel cells [20]. Fig.2 shows the voltammogram results of different scan rates for Mn+2, +3 and Mn+3, +4 containing the information about electron transfer, kinetics and transport properties of electrolysis reaction. The current was measured as a function of the linear potential applied. Such current variation as a result of the varied electrode potential can provide valuable insight into the reactions that occur at the electrode surface. The electrochemical behavior of MnSO4 was established by cyclic voltammetry (CV) for oxidation and reduction at a MnO2 nanoparticle electrode in distal water at scan rates that ranged from 0.01 to 0.2 V/s at a potential range of 2 to -2 V. At scan rate (ʋ) 0.1 V/s. As an example, two oxidation peaks were clearly obtained for Mn+2. +3 and Mn+3, +4. Oxidation peaks were obtained at Epox1 = -0.732 V, Epox2 = -1.040 V, corresponding to the anodic peak current Ipox1 = 1.7 × 10-4, Ipox2 = 4.890 × 10-4 connectivity. And single reduction peak was obtained at Epred = -0.526 V, corresponding to the anodic peak current Ipred = -1.05 × 10-4 A. Fig. 3 shows the response for ion selective electrode to manganese ion in multi-vitamins in black curve and no response in other treatments used, which means MnO2 nanoparticles carbon paste electrode was ion selective electrode for Mn+ ion. Table 1 refers to the result of voltammograms of MnO2 nanoparticles electrode in the presence of 1 ppm of MnSO4 solution at different scan rates of 0.01, 0.02, 0.05, 0.1 and 0.2 V/s, respectively.
Table 1 Result of voltammograms of MnO2 nanoparticles electrode in the presence of 1 ppm of MnSO4 solution at different scan rates of 0.01, 0.02, 0.05, 0.1, and 0.2 V/s, respectively
|
Fpred (A, V/s½, 10-4) |
Ipred (A,10-4) |
Fpoxd2 (A, V/s½, 10-4) |
Ipoxd2 (A,10-4) |
Fpoxd (A, V/s½,10-4) |
(A,10-4) |
√ʋ (V/s)1/2 |
ʋ (V/s) |
|
-79.980 |
-7.998 |
21.800 |
2.180 |
65.870 |
6.587 |
0.100 |
0.01 |
|
-67.531 |
-9.522 |
19.360 |
2.730 |
64.510 |
9.096 |
0.141 |
0.02 |
|
-55.022 |
-12.270 |
16.950 |
3.780 |
43.726 |
9.751 |
0.223 |
0.05 |
|
-46.507 |
-14.650 |
15.223 |
4.890 |
36.603 |
11.530 |
0.315 |
0.1 |
|
-57.091 |
-25.520 |
23.266 |
10.400 |
47.718 |
21.330 |
0.447 |
0.2 |
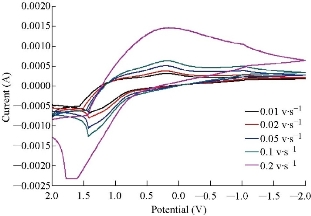
Fig. 2 Cyclic voltammograms of MnO2 nanoparticle electrode in the presence of 0.1 M of MnSO4 solution at different scan rates.
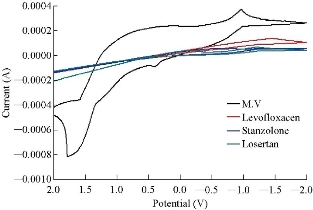
Fig. 3 Cyclic voltammograms of MnO2 nanoparticle carbon paste electrode and in 0.1 M multi-vitamins solution at the scan rate 0.1 V/s.
Temperature dependence studies on the electro-oxidation of MnO2 nanoparticle electrode
A study of the electro-oxidation on electrodes was investigated by CV, in the temperature range between 15 °C and 35 °C. Two mechanism of cyclic voltammetry appeared in voltammograms. Quesi and irreversible cyclic voltammetry and the kinetic and thermodynamic parameters calculated, after understanding the transported of two electrons by seeing that the curve shifting to the lift and slope of curve was also increased. This phenomena can be understood as the reaction took place on the electrode surface, the temperature of which was growing and the solvent was maintained at the temperature [21]. Fig. 4 and Table 2 demonstrate the results that the working electrode showed clear signal with the beaks shifting for more negative when increasing the scan rate. And from the slope between the lnD and 1/T K in Fig. 5, the ∆Ea was calculated to refer to the physical reaction accruing on the surface electrode and endothermic reaction when -∆H and ∆S referred to increased entropy when increasing the temperature. By using Matsuda and Ayabe equation, the diffusion coefficient was calculated, and by using α = nFʋ/RT equation, the transfer coefficient was calculated. Also, by using K = koe-anf(E-E0) equation, the rate constant reaction was calculated, where ko = 0.46 × ʋ1/2, and the value of ko was greater than 1 cm/s. The data were not corrected [22], and the result showed first order reaction on the surface of electrode. All the results are shown in Table 3.
Table 2 I-E data voltammograms for MnO2 nano electrode with different temperatures of 15, 20, 25, 30 and 35 oC, with diffusion coefficient for each step
|
Ln D |
D. coefficient (cm2/s) |
∆E = Epa2 - Epc/2 |
E = Epa - Epc |
Ipoxd A (10-4) |
Epc |
Ipoxd2 (A, 10-4) |
Epa2 |
Ipoxd1 (A, 10-4) |
Epa |
1/T 10-3 |
Temperature (K) |
|
-29.12 |
2.241 - 10-13 |
-1.190
|
-1.144 |
17.10 |
1.504 |
11.401 |
-0.877 |
7.520 |
0.360 |
3.47 |
288 |
|
-28.94 |
2.678 - 10-13 |
- 1.012 |
- 0.963 |
12.80 |
1.323 |
12. 420 |
-0.701 |
7.550 |
0.360 |
3.41 |
293 |
|
-29.54 |
1.447 - 10-13 |
-0.887 |
-0.661 |
9.560 |
1.090 |
9.099 |
-0.665 |
6.690 |
0.429 |
3.33 |
298 |
|
-29.422 |
1.666- 10-13 |
- 1.078 |
- 0.980 |
15.100 |
1.370 |
9.731 |
-0.786 |
7.560 |
0.390 |
3.30 |
303 |
|
-29.657 |
1.318- 10-13 |
-1.089 |
-0.988 |
13.100 |
1.321 |
8.625 |
-0.858 |
7.570 |
0.331 |
3.32 |
303 |
Table 3 Kinetic parameters of MnO2 nanoparticles electrode
|
Type of electrode |
∆E (kJ/mol) |
∆H (kJ/mol) |
∆G (kJ/mol) |
∆S (kJ/mol) |
K (s-1, 10-5) |
Do × 10-7 |
Α |
K0 |
|
MnO2 nanoelectrode |
-68.92 |
-6.79 |
-95.3 |
0.297 |
5.36 |
1.00 |
0.1 |
0.14 |
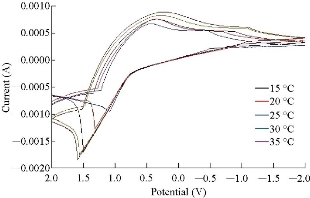
Fig. 4 Effect of temperature on the cyclic voltammograms on MnO2 nanoparticles electrode with different temperatures of 15, 20, 25, 30 and 35 oC, respectively.
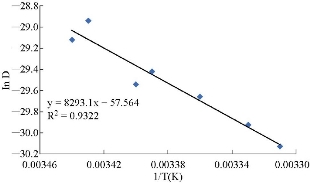
Fig. 5 Ln D - 1/T plot for the calculation of Ea energy for MnO2 nanoparticles electrode.
Calibration plots and determination of Mn ion at MnO2 nanoparticle carbon paste electrode
CV was used to obtain the analytical features of the method such as linear ranges of the calibration plots, and to detect the limits for Mn ion. The capability of separating the electrochemical response of Mn2+ by MnO2 nanoparticle modified electrode was studied. Therefore, CV was used for the simultaneous separation of species based on its superior elimination of the capacitive background current. Analytical experiments were carried out. The electrochemical response of Mn by MnO2 nanoparticle modified carbon paste electrode was studied by varying concentration of MnSO4 pure stock solution, Fig. 6 and 7 show the calibration curve by CV obtained in 0.5 - 4.5 ppm. It can be clearly seen that the response of the MnO2 nanoparticle electrode to Mn concentration. And by using the equation w.t = (ppm × M.wt of Mn/M.wt of drug) / (10 × V), where w.t refers to weight, ppm refers to concentration, M.wt refers to molecular weight, and V refers to volume. All results suggested that the MnO2 electrode was more sensitive, speedy, economical, clearer and safer than the colorimetric and atomic analyzer for determination of trace amount in drug component. Table 4 and 5 show the results of stock solution and real sample of copper (Hasanl A-Z Vital, Germany) containing vitamins.
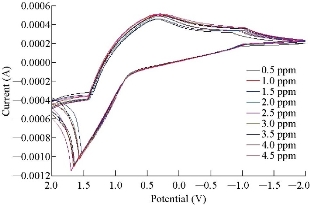
Fig. 6 Cyclic voltammograms of calibration curve by MnO2 nanoparticles electrode for MnSO4 solutions.
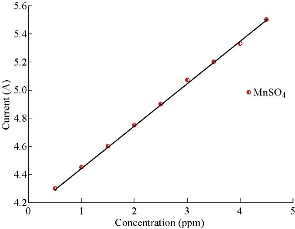
Fig. 7 Calibration curve by MnO2 nanoparticle electrode for MnSO4 solutions.
Table 4 Results determined of real sample of drug by deferent method
|
RSD (%) |
Founded (ppm) |
Linear range |
Analyte, Mn (mg) |
Method |
|
62.0 |
0.62 |
10-3 - 10+3 ppm |
1 |
Cyclic voltammetry |
|
74.5 |
1.49 |
2 |
||
|
79.6 |
1.99 |
2.5 |
||
|
21.0 |
0.21 |
0.08 - 5 ppm |
1 |
Coulometry |
|
55.0 |
1.10 |
2 |
||
|
79.2 |
1.99 |
2.5 |
||
|
73.0 |
0.73 |
0.1 - 10+3 ppm |
1 |
Atomic radiation |
|
81.0 |
1.62 |
2 |
||
|
84.0 |
2.10 |
2.5 |
Table 5 Results determined of stock solution by deferent method
|
RSD (%) |
Founded (ppm) |
Linear range |
Analyte, Mn (mg) |
Method |
|
87.0 |
0.87 |
10-3 - 10+3 ppm |
1 |
Cyclic voltammetry |
|
95.0 |
1.90 |
2 |
||
|
95.0 |
2.31 |
2.5 |
||
|
81.0 |
0.81 |
0.08 - 5 ppm |
1 |
Coulometry |
|
93.5 |
1.87 |
2 |
||
|
88.0 |
2.20 |
2.5 |
||
|
90.0 |
0.97 |
0.1 - 10+3 ppm |
1 |
Atomic radiation |
|
93.5 |
1.88 |
2 |
||
|
96.0 |
2.39 |
2.5 |
Conclusions
Nano MnO2 electrode is a highly sensitive and selective for small changes in the current passing through its solution when it becomes an electrode. Therefore, in this study, it was used to prepare the electrode and to study its physical and chemical properties and applied to measure the small concentrations of drugs containing manganese, the results were highly accurate in standard solutions and in real sample when compared to common methods such as atomic analyzer and colorimetric method. Results referred to the development in electronic conductivity when MnO2 nanoparticles were prepared and ion selective electrode was made with special properties and easy use.
References
Copyright© Emad Salaam Abood, Mothana Salih Mashkoor, and Amer Mousa Jouda. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.