Research Article
Preparation and Characterization of Methotrexate Loaded Polymeric Nanoparticles by Nanoprecipitation Technique
Ayesha Siddiqua Gazi, Abbaraju Krishnasailaja *
RBVRR Women’s College of Pharmacy, Osmania University, Barkatpura, Hyderabad, India.
* Corresponding author. E-mail: asharpai2013@gmail.com
Received: Sep. Apr. 8, 2019; Accepted: Nov. 18, 2019; Published: Nov. 18, 2019
Citation: Ayesha Siddiqua Gazi, Abbaraju Krishnasailaja, Preparation and Characterization of Methotrexate Loaded Polymeric Nanoparticles by Nanoprecipitation Technique. Nano Biomed. Eng., 2019, 11(4): 351-360.
DOI: 10.5101/nbe.v11i4.p351-360.
Abstract
The aim of this study was to develop and characterize methotrexate-loaded polymeric nanoparticles by nanoprecipitation technique. Eudragit® S 100 and ethyl cellulose were employed to develop methotrexate-loaded nanoparticles by nanoprecipitation technique. Six different formulations (f1, f2, f3, f4, f5 and f6) were prepared with each polymer by varying the drug to polymer ratios (1:1, 1:1.5, 1:2, 1:3, 1.5:1 and 2:1). Dimethyl sulfoxide (DMSO) was used as a solvent and Tween® 20 as a surfactant. Among the six formulations of polymeric nanoparticles prepared by nanoprecipitation technique, F4 formulation was considered as a best formulation with each polymer. Based on comparison results of mean particle diameter, zeta potential, drug content and entrapment efficiency, Eudragit® S 100 was considered to be the most suitable polymer for preparation of methotrexate-loaded nanoparticles by nanoprecipitation technique. Based upon the evaluation studies, the best formulation was characterized for scanning electron microscopy (SEM), particle size, zeta potential and anti-cancer activity in MCF-7 cell line by MTT assay.
Keywords: Methotrexate; Polymeric nanoparticles; Nanoprecipitation technique
Introduction
A conventional preparation like solutions, suspensions or emulsions for drug delivery has certain restrictions like high dose and low bioavailability, intolerance, instability. They show some changes in the blood plasma drug levels and do not provide sustained effect. Due to the attendance of acidic and basic medium in the body, it is essential that every drug should reach the target site without any alteration in its physical and chemical properties [1, 2]. The major focus on Novel drug delivery systems during the past two decades is to improve the therapeutic efficacy and safety profile of the drug substances. Among all the colloidal systems, nanoparticles hold promise as drug delivery through various routes due to their greater stability and easier manufacturing ability. And also since many years, there has been a shift in drug delivery research from micro to nanoscale. Thus, nanotechnology is evolving as a promising field in medicine that shows significant therapeutic benefits. These systems are used for specific drug delivery, controlled drug delivery and also for the improvement of bioavailability of the hydrophobic drugs [3, 4]. Methotrexate (MTX), a dihydrofolate reductase inhibitor, is one of the most widely used drugs for the treatment of various neoplastic diseases. It is used in the treatment of certain cancers like breast cancer, skin and lung cancer. Clinical studies have revealed that the curative effect of MTX tablet regarding cancers was limited due to its toxic dose-related side effects to normal cells, nephrotoxicity, immunosuppressant, bone marrow suppression, and ulcerative stomatitis, acute and chronic hepatotoxicity and also due to the drug resistance of the tumor cells. Hence, there is a need to develop methotrexate nanoparticles in order to minimize the adverse effects associated with the MTX tablet dosage form [5, 6]. Nanoprecipitation is also called solvent displacement or interfacial deposition. It is considered as one of the first developed techniques used for the encapsulation of drug molecules [7]. This technique was developed by Fessi et al. (1989). Although, several techniques have been used for the preparation of submicron particles from preformed polymers, nanoprecipitation is regarded as a quite simple and reproducible technique that allows the obtaining of submicron-sized polymer particles [8, 9]. In the present study, attempts have been made to prepare methotrexate nanoparticles by using Eudragit® S 100 and ethyl cellulose (EC) as a polymer as these are low cost polymer and can formulate the methotrexate nanoparticles in academic research.
Experimental
Materials
Drug: Methotrexate, gift sample from Mac-Chem Products (India) Pvt. Ltd., Mumbai.
Polymers: Eudragit® S 100, ethyl cellulose obtained from SD Fine Chem. Limited, Mumbai.
Solvent: DMSO, acetone obtained from SD Fine Chem. Limited, Mumbai.
Stabilizer: Tween® 20 obtained from SD Fine Chem. Limited, Mumbai.
Methods
Methotrexate loaded polymeric nanoparticles were prepared by nanoprecipitation technique. Eudragit® S 100 and ethyl cellulose was selected as a polymer for this technique. DMSO was used as a solvent [10].
Nanoprecipitation
Six different formulations (F1, F2, F3, F4, F5, and F6) were prepared by varying the drug to polymer ratio. Weighed quantities of Methotrexate and Eudragit® S100 / ethyl cellulose were dissolved in 10 mL of dimethyl sulphoxide. (Ethyl cellulose was dissolved in acetone before adding DMSO). The organic phase was then added to 25 mL of aqueous phase containing 0.1% Tween® 20 surfactant with continuous stirring at 500 rpm. The appearance of precipitate was considered as the end point. After the attainment of endpoint, the solution was kept for stirring for 5 hours. The formed precipitate was separated from the solution by means of vacuum rotary evaporator. The obtained precipitate was air-dried to completely remove the moisture content [11, 12].
Table 1 Formulation ratios of methotrexate loaded ES 100 / ethyl cellulose nanoparticles
|
Formulation |
Ratio of drug to polymer used |
Optimized parameters |
|
F1 |
1 : 1 |
|
|
F2 |
1 : 1.5 |
DMSO |
|
F3 |
1 : 2 |
0.1% v/v Tween® 20 |
|
F4 |
1 : 3 |
500 rpm |
|
F5 |
1.5 : 1 |
5 h |
|
F6 |
2 : 1 |
|
Evaluation studies [13]
The obtained formulations of technique are evaluated for the following parameters.
Determination of drug content
Free drug of the formulations was first determined in the supernatant by choosing a solvent in which only the free drug got dissolved and not the other ingredients .To determine the drug content, 50 mg drug equivalent to formulation was weighed accurately and transferred into 100 mL beaker containing 50 mL of DMSO. The solution was stirred at 700 rpm for 3 h by using magnetic stirrer. The resultant solution was filtered and the amount of the drug in the filtrate was estimated after suitable dilution by UV spectrophotometer at 303 nm [14, 15].
Entrapment efficiency [16]
Entrapment efficiency indicates the amount of drug encapsulated in the formulation. The method of choice for entrapment efficiency determination is separation of free drug by ultracentrifugation, followed by quantitative analysis of the drug from the formulation. The samples were centrifuged by using ultracentrifuge at 17000 rpm for 40 min.
Percentage entrapment efficiency may be calculated from the following formula:
![]()
Loading capacity [17]
It indicates the capacity of the polymer to load a drug.
![]()
![]()
In-vitro drug release kinetics [18, 19]
In-vitro drug release studies were conducted by means of orbitary shaker. 50 mg of each accurately weighed formulation was transferred into 250 mL conical flask containing 50 mL pH 7.4 phosphate buffer. They were kept in an orbitary shaker at 100 rpm maintained at 37 °C. Aliquots of 2 mL buffer were withdrawn at predefined time intervals and the medium was replaced with same volume of buffer. This study was carried out for 12 h, and the amount of drug release was estimated by determining the absorbance at 303 nm using Elico UV spectrophotometer.
Characterization of nanoparticles [20]
Particle size analysis and zeta potential measurement [21, 22]
The average particle size and size distribution of MTX polymeric nanoparticles were determined by dynamic light scattering (DLS), using Malvern Zetasizer. The zeta potential (surface charge) which indicates the stability of the nanoparticle’s can be defined as electrokinetic potential that is determined by electrophoretic mobility. Samples were prepared by diluting with water and corresponding zeta potential was measured using Malvern Zeta Sizer.
Determining the size and morphology of the nanoparticles [23, 24]
Scanning electron microscopy (SEM) was used to determine the shape, size and surface morphology of the MTX polymeric nanoparticles. Nanoparticulate suspension is made to obtain photomicrographs using SEM.
In vitro cytotoxicity assay
Aim
To determine the Anti-cancer activity of test compounds in vitro by MTT Assay.
Materials and methods
Dulbecco's modified Eagles medium (DMEM), MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide], trypsin, and EDTA phosphate buffered saline (PBS) were purchased from Sigma Chemicals Co. (St. Louis, MO). Fetal bovine serum (FBS) was purchased from Gibco, 25 cm2 and 75 cm2 flask and 96 well plated purchased from Eppendorf India [25, 26].
Procedure
Cell viability was evaluated by the MTT Assay with three independent experiments with six concentrations of compounds in triplicates. Cells were trypsinized and perform the tryphan blue assay to know viable cells in cell suspension. Cells were counted by haemocytometer and seeded at density of 5.0 × 103 cells / well in 100 μL media in 96 well plate culture medium and incubated overnight at 37 ℃. After incubation, take off the old media and add fresh media 100 µL with different concentrations of test compound in the wells in 96 plates. After 48 h., Discard the drug solution and add the fresh medic with MTT solution (0.5 mg/mL) was added to each well and plates were incubated at 37 ℃ for 3 h. At the end of incubation time, precipitates are formed as a result of the reduction of the MTT salt to chromophore formazan crystals by the cells with metabolically active mitochondria. The optical density of solubilized crystals in DMSO was measured at 570 nm on a microplate reader. The percentage growth inhibition was calculated using the following formula and concentration of test drug needed to inhibit cell growth by 50% values is generated from the dose-response curves for each cells using with origin software [27, 28].
![]() × 100
× 100
Results and Discussion
The obtained formulations were evaluated for the above mentioned parameters and the results are discussed as follows.
Evaluation results of methotrexate-Eudragit® S 100 nanoparticles
Drug content
All the prepared formulations of MTX loaded Eudragit® S100 nanoparticles were evaluated for drug content. Drug content of the formulations, F1 - F6 was found to be 72±2%, 72.6±3%, 80.4±2%, 93.1±3%, 77.2±2%, and 62.6±2%, respectively. These results revealed that all the formulations showed good drug content and F4 formulation showed the highest percentage of drug content, i.e.; 93.1±3%.
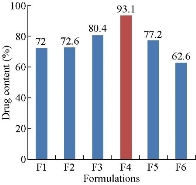
Fig.1 Drug content of methotrexate-Eudragit® S100 nanoparticles.
Entrapment efficiency
The entrapment efficiency of all the formulations, i.e. F1 - F6, was determined to be 77.09±2%, 79.19±3%, 80.06±2%, 82.5±3%, 72.75±1% and 74.9±2%, respectively. As the amount of polymer was increased from F1 to F4 formulation, the encapsulation efficiency was increased. However, on increasing the drug concentration, there was decrease in entrapment efficiency that could be due to an inadequate amount of polymer present in the system being insufficient to entrap the drug inside the matrix. Thus, the optimum drug encapsulation was found to be for F4 formulation showing i.e.; 82.5%.
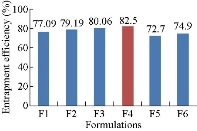
Fig. 2 Drug entrapment efficiency of methotrexate-Eudragit® S100 nanoparticles.
Loading capacity
Loading capacity of all the formulations, F1 - F6 was found to be 26.9%, 27.4±1%, 25.04±2%, 16.6±1%, 33.3±2% and 29.58±2%, respectively. By comparison, F5 formulation showed higher loading capacity than other formulations.
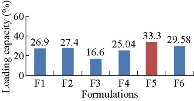
Fig. 3 Loading capacity of Methotrexate-Eudragit® S 100 nanoparticles.
In-vitro drug release studies
The in-vitro drug release studies were performed for all the formulations of MTX loaded ES 100 nanoparticles in 7.4 pH phosphate buffer conducted by means of orbitary shaker (Orchid, Scientifics) for a time period of 12 h. Among all the formulations, the drug release from F4 formulation was good and the release was sustained up to 12 h, i.e.; 89.8±3% in 12 h.
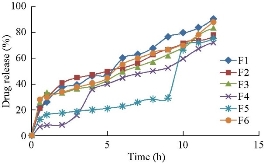
Fig. 4 In-vitro drug release profile of methotrexate-Eudragit® S 100 nanoparticles.
Evaluation results of methotrexate-ethyl cellulose nanoparticles
Drug content
Drug content of the formulations F1 - F6 was found to be 68.9±2%, 69.02±2%, 70.6±3%, 75±3%, 70.2±2% and 82.3±2%, respectively. These results revealed that all the formulations showed good drug content and F6 formulation showed the highest percentage of drug content, i.e.; 82.3±2%.
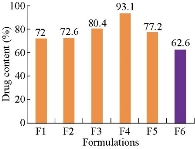
Fig. 5 Drug content of methotrexate-ethyl cellulose nanoparticles.
Entrapment efficiency
Entrapment efficiency of all the formulations, F1 - F6 was determined to be 61.4±2%, 65.9±2%, 74.2±3%, 79.3±2%, 59.5±2% and 53.4±3%, respectively.
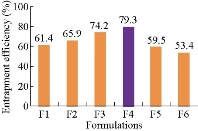
Fig. 6 Drug entrapment efficiency of methotrexate-ethyl cellulose nanoparticles.
Loading capacity
Loading capacity of all the formulations, F1 - F6 was found to be 21.54±2%, 20.57±2%, 19.25±3%, 18.1±2%, 24.6±2% and 29.7±2%, respectively. By comparison, F6 formulation showed higher loading capacity than the other formulations.
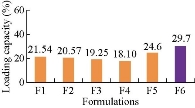
Fig. 7 Loading capacity of methotrexate-ethyl cellulose nanoparticles.
In-vitro drug release studies
Among all formulations F4 (1:3) formulation showed maximum drug release of 90.05±2% in a time period of 12 h as shown in Fig. 8.
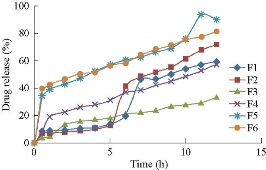
Fig. 8 In-vitro drug release profile of methotrexate-ethyl cellulose nanoparticles.
Average particle size
Size distributions of the prepared nanoparticles along the mean diameter was measured using particle size analyser. Average particle size of the prepared drug loaded Eudragit® S 100 nanoparticles was recorded. The minimum value was found for F4 formulation as of 704.3 nm (Fig. 9).
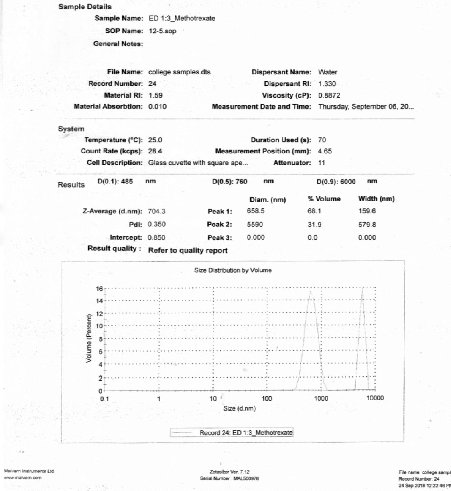
Fig. 9 Particle size analysis of F4 formulation.
Zeta potential
Zeta potential of the prepared drug loaded Eudragit® S 100 nanoparticles was measured using zeta sizer. Nanoparticles of F4 formulation showed higher stability, bearing a value of -21.3 mV.

Fig. 10 Zeta potential analysis of F4 formulation.
Average particle size and zeta potential
The size distributions of the prepared nanoparticles along the mean diameter were measured using particle size analyser. The average particle size of the prepared drug loaded ethyl cellulose nanoparticles was recorded. The minimum value was found for F4 formulation as of 622.6 nm (Fig. 11 and 12).
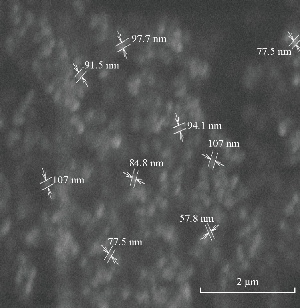
Fig. 11 SEM of F4 formulation.
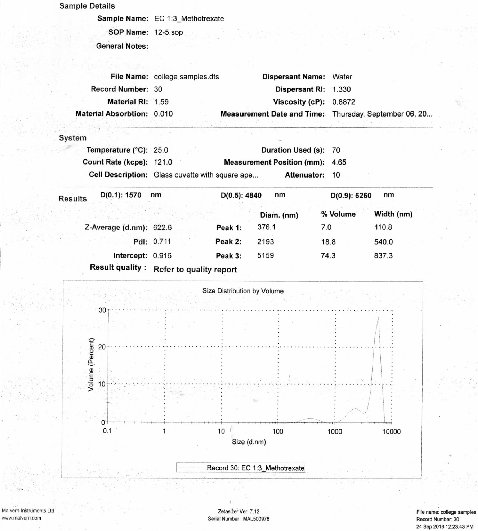
Fig. 12 Particle size analysis of F4 formulation.
Zeta potential
Zeta potential of the prepared drug loaded ethyl cellulose nanoparticles was measured using zeta sizer. Nanoparticles of F4 formulation showed higher stability, bearing a value of – 23.3 mV.
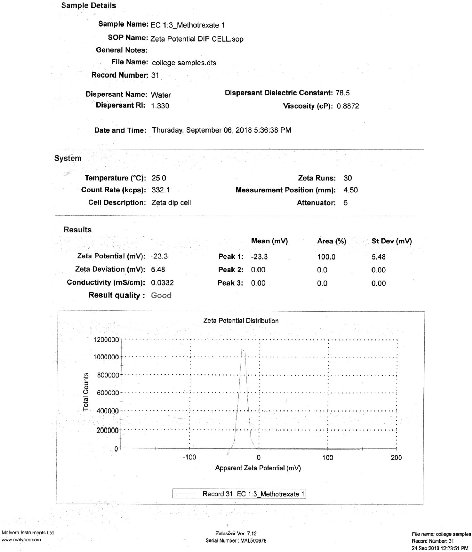
Fig. 13 Zeta potential analysis of F4 formulation.
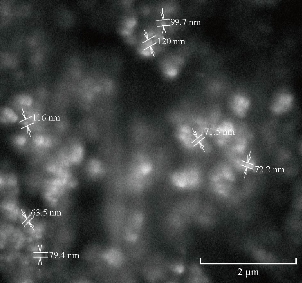
Fig. 14 SEM of methotrexate-ethyl cellulose F4 formulation.
In-vitro cytotoxicity assay
Based on the characterization and evaluation studies of the best formulations prepared by nanoprecipitation technique, MTX-EC (1:3) was considered as a best formulation and determine for the anticancer activity on MCF-7 cell line by MTT assay. Cisplatin was taken as a standard and its IC50 value was observed to be 4.7 μM. The IC50 value for the given formulation (MTX-EC 1:3) was found to be 74.65 µg.
Table 2 Cytotoxicity effect of F4 formulation (methotrexate- ethyl cellulose 1:3) on the growth of MCF-7 cell line
|
Concentration (µg) |
OD at 570 |
Inhibition (%) |
Viability (%) |
|
5 |
0.281 |
20.62 |
79.38 |
|
10 |
0.175 |
50.56 |
49.44 |
|
25 |
0.177 |
50 |
50 |
|
50 |
0.167 |
52.82 |
47.18 |
|
75 |
0.163 |
53.95 |
46.05 |
|
100 |
0.192 |
45.76 |
54.24 |
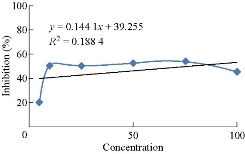
Fig 15 Cytotoxicity effect of methotrexate-EC (1:3) on the growth of MCF-7 cell line.
Table 3 IC50 value of methotrexate-EC 1:3 formulation
|
S. No. |
Sample name |
IC50 (µg) |
|
MCF7 |
||
|
1 |
ED (1:3) |
74.65 |
Discussion
In the present study, attempts have been made to prepare methotrexate nanoparticles by using Eudragit® S 100 and ethyl cellulose as a polymer as these are low cost polymer and can formulate the methotrexate nanoparticles in academic research. Methotrexate acts as anti-cancer drug through its anti-metabolic effect by interfering with DNA and RNA growth by substituting for the normal building blocks of RNA and DNA. MTX is one of the most commonly used chemotherapeutic agent for human malignancies. Six formulations (1:1, 1:1.5, 1:2, 1:3, 1.5:1 and 2:1) were prepared with each polymer by varying the concentrations of drug and polymer. Out of six formulations of Eudragit® S 100, F4 formulation was found to be the best formulation with drug content of 93.1%, entrapment efficiency of 82.5%, loading capacity of 25.04%, mean particle diameter of 704.3 nm, zeta potential value of -21.3 mV. In-vitro drug release data showed 89.8% of drug release controlled up to 12 h. Out of six formulations of ethyl cellulose, F4 formulation was found to be the best formulation with drug content of 75%, entrapment efficiency of 79.3%, loading capacity of 18.1%, mean particle diameter of 622.6 nm, zeta potential value of -23.3 mV. In-vitro drug release data showed 90% of drug release controlled up to 12 h. In-vitro drug release studies and its data analysis have proven that all the formulations showed a sustained release that followed zero order and fickian type of diffusion pattern. Based on the characterization and evaluation parameters, F4 (1:3) formulation using Eudragit® S 100 as a polymer was found to be the best formulation by nanoprecipitation technique. This formulation was considered for determining anticancer activity by MTT assay in MCF-7 breast cancer cell line. From the results it was found that the formulation exhibited anticancer activity at an IC50 value of 74.65 µg.
Conclusions
Both polymers were compared for the characterization and evaluation parameters. By comparison, Eudragit® S100 was found to be a better polymer over ethyl cellulose for the preparation of methotrexate nanoparticles because of its smaller mean particle diameter (704.3 nm), higher stability (-21.3), greater entrapment efficiency (82.5%), and anti-cancer activity at an IC50 value of 74.65 µg.
Acknowledgements
The authors would like to thank RBVRR Women’s College of Pharmacy, Principal Dr. M. Sumakanth for providing us funds for the work. The authors would like to acknowledge Mrs. K. Sumalatha and Mrs. D. Suvarna for providing us technical assistance.
References
Copyright© Ayesha Siddiqua Gazi, Abbaraju Krishnasailaja. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.