Research Article
Effect of Antimonial Therapy on Levels of (TNF-α and IL-1β) Cytokines in Cutaneous Leishmaniasis Patients in Iraq
Hasan Raheem Khudhur 1, Ahmed Abbas Hasan 2, Rawaa AddayAli 3
1 Ministry of Education, Iraq.
2 College of Health and Medical Techniques, Kufa, Al-Furat Al-Awsat Technical University, 31003 Al-Kufa, Iraq.
3 AL-Qasim Green University, College of Veterinary Medicine, Microbiology Department, Iraq.
* Corresponding author. E-mail: hassanraheem1990@gmail.com
Received: Jul. 25, 2019; Accepted: Nov. 11, 2019; Published: Nov. 11, 2019
Citation: Hasan Raheem Khudhur, Ahmed Abbas Hasan, and Rawaa Adday Ali, Effect of Antimonial Therapy on Levels of (TNF-α and IL-1β) Cytokines in Cutaneous Leishmaniasis Patients in Iraq. Nano Biomed. Eng., 2019, 11(4): 340-346.
DOI: 10.5101/nbe.v11i4.p340-346.
Abstract
This study aimed to study the effective antimonial therapy on circulating levels proinflammatory cytokines, and their effect on susceptibility to cutaneous leishmaniasis (CL) infection in the Iraqi population. Fifty CL patients were treated with pentavalent antimonial salts (pentostam) for 7 weeks. Leishmania species were identified by Nested-Polymerase chain reaction method, and in all the cases the strains corresponded to Leishmania major. Circulating plasma levels of the proinflammatory cytokines interleukin-1 and tumor necrosis factor-alpha were determined for CL patients and healthy subjects before and during 7 weeks after the treatment were started. Concentrations were detected by enzyme-linked immunosorbent assay technique using a quantitative sandwich enzyme immunoassay technique. Proinflammatory cytokines significantly increased after 7 days postinfection compared to levels in the pretreatment patients. It was clearly recorded in the present study that the level of interleukin-1 and tumor necrosis factor-alpha in the serum of CL patients was responsively increasing with the antimonial therapy dose.
Keywords: Cutaneous leishmaniasis; Antimonial therapy; TNF-α and IL-1β; Iraq
Introduction
Cutaneous leishmaniasis (CL) is a parasitic disease transmitted by sand flies and caused by obligate intra-macrophage protozoa [1, 2]. CL occurs each year, and more than 90% of the cases occur in five countries in the old word including Afghanistan, Algeria, Iran, Iraq, and Saudi Arabia, and two countries in the new world including Brazil and Peru [3]. Leishmania major and Leishmania tropica are considered as common causes of CL in Iraq [4]. The principal effective mechanism mediating parasite elimination is the activation of macrophages by proinflammatory cytokines, and production of cytokines that activates macrophages correlates with healing responses [5-7]. The strongest evidence has come from laboratory models of protozoan infections. In malaria, toxoplasmosis, and leishmaniasis, to name just a few, the preferential production of proinflammatory cytokines results in increased synthesis of nitric oxide (NO) and reactive oxygen species which are involved in host protection processes by direct toxicity on parasites or by inhibiting parasite growth [8-9]. The tissue-resident macrophages are the definitive host cells for parasite survival and replication. In addition, classical activation of infected macrophages by tumor necrosis factor (TNF) stimulates the production of inducible nitric oxide (NO), an enzyme that catalyzes l-arginine to generate nitric oxide. NO is a powerful cytostatic and cytotoxic molecule, and plays a major role in killing many intracellular parasites, including Leishmania; thus, in leishmaniasis, macrophages play a dual role, representing an important cell population responsible for the killing of the parasites and also the major site of parasite replication [10]. TNF-α is produced by mononuclear phagocyte, fibroblast, B and T cell; macrophages participates in the production of TNF-α; T cell induces macrophages to produce NO, which causes control or killing of parasites; TNF-α secreted by macrophages also mediates in the secretion of nitric oxide as well as activation of macrophages and parasite killing [11]. IL-1β is well known for that cytokines are considered to play a key role in the inflammation process. The proinflammatory cytokine is important in that it mediates in the secretion of nitric oxide as well as activates monocytes, macrophages and neutrophils. On the other hand, it induces Th1 and Th17 adaptive cellular responses [13, 14]. The development of safe, affordable and effective drugs for the treatment of leishmaniasis is still urgently required. The fact that recovery from infection leads to resistance against further infection suggests the production of a successful vaccine is feasible; however, although recent investigations into the possibility of live attenuated vaccines have been promising, no vaccine is currently available [15]. CL is considered as a self-healing disease; hence, rapid treatment remains important to avoid unattractive scars and parasite dissemination, i.e. nodular lymphangitis and MCL. At present, no single optimal treatment exists for CL [2, 16]. The objective of treatment of CL is to improve the cosmetic effect of scar and to shorten the duration of the disease. There are many factors affecting the treatment of CL, such as clinical presentation, duration of disease, number, size, and site of lesion and presence of secondary infection. Pentavalent antimonial (Pentostam) remains the drug of choice for the treatment of all types of leishmaniasis; it is thought to work by inhibition of adenosine triphosphate synthesis (ATP) [17, 18]. Pentostam dose per lesion is 0.2-0.4 mL (100 mg/mL) or 15-20 mg/kg/day for 15-20 times every other day, more or less depending on the lesion and its response to treatment [19, 20]. Pentostam can be given intramuscularly (IM) or intravenously (IV) depending on the progress and stage of the lesion [17]. Though Pentostam is effective against some forms of leishmaniasis, increasing levels of resistance have been reported [21]. Pentavalent antimonials remain the drugs of choice in the treatment of all forms of Leishmania infection in spite of their reported toxicity, difficulty in administration, and high cost. However, it is not known whether the antimonial compound acts directly on the parasite or by activating the macrophage or other components of host defense systems which subsequently exert an effect on the Leishmania parasite [22]. Several studies have demonstrated that there is an important immunological component in response to antimonial therapy [23, 24]. Murray et al. reported that cytokine production is involved in the healing process following antimonial treatment [25]. It has also been demonstrated that antimonial salts potentiate oxidant production in murine visceral leishmaniasis and in human blood [26]. However, the association between pro-inflammatory cytokines and antimonial therapy in CL patients is not known. Our main objective in this study was to understand the effects of antimonial compounds on the circulating levels of some proinflammatory cytokines, namely, interleukin-1β, and tumor necrosis factor-alpha (TNF-α), before and during therapy for patients with CL.
Experimental
Subjects and study design
The study was conducted at Al-Hussein Teaching Hospital and specialized center of sensitivity in Al-Muthanna Province, Iraq. This province is located in the southeastern region and is an area where leishmaniasis is endemic. A total of 50 cases of suspected cutaneous leishmaniasis and 35 healthy individuals aging between 8 to 50 years from the same area were included in this study during the period from August 2018 till the end of January 2019. The patients were complaining of a skin lesion in an exposed part of the body mostly in the face, leg and arm. Cases diagnosed clinically by a special dermatologist as cutaneous leishmaniasis and confirmed as CL patients based on clinical symptoms and direct Giemsa-stained smear, prepared with material aspirated from borders of skin lesions. Small amount of aspirated fluid was taken and smeared on a clean glass microscope slide, left to dry, and then fixed using 100% absolute methanol for 30 sec and left to dry again. The microscopic slide was stained with Giemsa stain for 20 min, rinsed with tap water and dried, and then examined under oil immersion lens of the light microscope. Amastigote was diagnosed as round or spherical shape with distinctive kinetoplast, in which case it was declared positive. When no amastigote was seen after 15 min of inspection, the smears were declared negative [27]. Genomic DNA was extracted from wound lesion fluid by using AccuPrep®Genomic DNA extraction kit (Bioneer. Korea) according to company instruction. The characterization of the causative parasite species and strains was conducted by Nected-PCR method; in all cases the strains corresponded to Leishmania major. Admission criteria for the patient group were no prior intralesional and no pregnancy and infection or systemic antimonial therapy. The criteria did not involve a restriction on the basis of sex or age. Patients with lesions of 6 months or greater duration were excluded from the study because of the possibility of spontaneous healing and immunity. The patients who had a single lesion smaller than 2 cm were also excluded from the study. Prior to blood sample collection, all patients and control subjects were fasted for 12 h in order to exclude dietary differences. 3 mL of blood was taken from CL patient at different time (before treatment, during treatment: after 7 days of dose injection, and including 7 doses divided on 7 weeks) into plain tube (serum tube). The blood samples were centrifuged at 4700 rpm for 5 min to obtain blood serum and then frozen at -20 ºC for screening of TNF-α, IL-1β cytokines levels were determined by ELISA technique using a quantitative sandwich enzyme immunoassay technique (ELISA kits for TNF-α and IL-1β by PeproTech Company, Germany). All tests were conducted according to company instructions. The results were calculated by ELISA reader (optical density at 405 nm immediately) and applied on a standard curve in order to sort out the concentration of the cytokine. After the first blood sample collection, the CL patients were cured with pentavalent antimonial (Fig. 1) at weekly intramuscular doses of 10 mg/kg for 7 weeks. Pentavalent antimonial treatment was given only for lesions larger than 2 cm in diameter, for multiple lesions, or for ulcerating lesions. The CL patients were checked monthly for healing or recurrence. Efficacy of antimonial therapy was defined as complete clinical healing of the lesion with the disappearance of edema, induration, or other signs of inflammation. Final healing was performed at the end of 4 months after therapy.
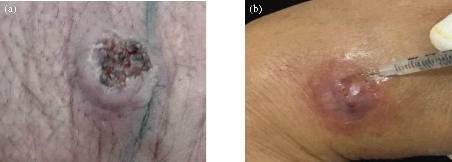
Fig. 1 CL lesion of (a) male patient with L. major; (b) injection of antimonial therapy dose (female patient with L. major).
Statistical analysis
Statistical analysis was conducted using SPSS 23. Determination of the statistical differences among different groups was performed by using Pearson's chi-squared test (ᵡ2), and mean cytokine levels were compared between groups using t-test. [29]. The probability of p ≤ 0.05 was considered to be statistically significant.
Results and Discussion
The results presented in this study were based on the analysis of a random sample of 50 cases with an established diagnosis of CL. Ages of the CL patients included in this study ranged between 8-30 years old. And there were more male patients (32; 64%) than female patients (18; 36%) in the present study. This is possibly because males have more exposure to infected vectors compared to females, due to the fact that a high percentage of males were working or sleeping in open areas (surfaces of houses) with less coverage of body [30]. Some studies have hypothesized that sex difference observed in some parasitic diseases can be attributed to hormonal effects; however, controversy still exists regarding the role of sex hormones in immune response. Behavioral factors that make males more exposed to the sand flies in the environments are probably equally or more important. Previous studies in Iraq revealed significantly more male infectious with CL than female, including in Baghdad [31], in Tikrit [32], in Al-Qadisiya [33, 34], in AL-Haweja city [35], and in Iran [36], while in Brazil it was shown that 71% of patients were male [37], higher than the rate of male in the present study 56.6%. It was found that the mean concentration of TNF-α increased gradually with the increasing of antimonial therapy dose (p < 0.001) (Table 1 and Fig. 2). The mean concentration of IL-1β level increased continuously with the increasing of antimonial therapy dose (p < 0.001) (Fig. 3). The mean concentration of TNF-α and IL-1β in patients has a significant increase in the patient groups in comparison with the control group. This increased expression may be due to an increased level of cellular activation or a relative increase in the number of cytokine-producing cells. The results were in agreement with those reported by Al-Aubaidi [38] in Iraq and by Kocyigit [28] in Turkey, where they found circulating proinflammatory TNF-α cytokine levels increased in patients with CL. Sodhi et al. [39] in India also found that IL-1β concentration in patients infected with leishmaniasis was higher than control groups. Another previous study in Iraq in 2018 found that cytokines (TNF-α and IL-1β) played an important role in the resolution of CL infection; their concentration in the patients’ serum of all age groups were increased in comparison with that observed in control groups. It was found that the mean TNF-α was significantly higher in patients as compared to control groups, 2.698 ± 0.122 ng/mL versus 0.414 ± 0.015 ng/mL respectively. In addition, the mean concentration of IL-1β interleukin significantly increased in patient groups in comparison to control subjects, 0.814 ± 0.054 ng/mL versus 0.482 ± 0.020 ng/mL respectively [40]. However, macrophage produces IL-1β which activates T-helper cells mediating the activation of macrophages to produce NO, resulting in killing or control of L. major parasites. The secretion of TNF-α by macrophages is sufficient to mediate the production of NO and the killing of L. major parasites [11]. In vitro studies with murine macrophages revealed that soluble factors secreted by activated T cells mediate the activation of macrophages to produce NO, resulting in killing or control of L. major parasites [11]. TNF-α has been shown to be a major NF-B-activating signal for macrophage activation [41]. Thus, the secretion of TNF-α by macrophages is sufficient to mediate the production of NO and the killing of L. major parasites [42]. It has also been demonstrated that TNF-α levels were higher in the sera of pretreatment CL patients [28]. Several suggestions have been made about the effects of antimonial therapy, and the generally accepted argument is that antimonial agents use pro-inflammatory cytokines to adequately stimulate macrophages [43]. We therefore investigated any possible association between antimonial therapy and pro-inflammatory cytokines in the present study. In this study, levels of TNF-α and IL-1β in the serum of CL patients were responsive and refractory to antimonial therapy; the result was shown to be significantly elevated with treatment dose (Fig. 2 and 3). The reason for higher concentrations of TNF-α and IL-1β in the serum of CL patients being responsive to treatment with sodium stibogluconate (Pentostam) lies generally in that Pentostam induces cytokines to activate macrophages [43]. Pentostam compounds might have essential effects that stimulate immunity components resulting in antimicrobial activity [44, 45]. Some studies refer to that cytokine secretion is important in the process of healing following treatment with Pentostam [26, 46]. The results of this study were in agreement with the findings by Kocyigit et al. [28] in Turkey and Al-Aubaidi [38] in Iraq. Also, Sodhi et al. [39] in India found the concentration of IL-1β in an animal was highly infected with L. donvani especially when treated with pentavalent antimonials. Sodhi et al. [47] demonstrated that IL-1β levels were significantly increased when Leishmania donovani-infected animals were treated with ammonium salts 14 days post-infection. Although they studied L. donovani-infected animals, their findings seemed to support the present study. It is thought that TNF-α and IL-1β levels increase as a part of host defense strategies and that the induction of the cytokines by antimonial therapy might be dependent on macrophage activation. Our results suggest that pro-inflammatory cytokines may play a crucial role in the resolution of CL infection. Pentavalent antimonial compounds may have immunostimulating effects which may be responsible for their antimicrobial activity.
Table 1 Association between the dose of treatment and serum TNF-α and IL-1β in patient groups.
|
Type |
TNF-α level (ng/mL) |
IL-1β level (ng/mL) |
|
Control (35) |
0.511 ± 0.035 |
0.212 ± 0.030 |
|
Patients before treatment |
2.289 ± 0.095 |
0.61 ± 0.042 |
|
Patients with dose1 |
2.338 ± 0.250 |
0.689 ± 0.039 |
|
Patients with dose 2 and 3 |
3.199 ± 0.134 |
1.19 ± 0.058 |
|
Patients with dose 4 and 5 |
4.251 ± 0.310 |
1.494 ± 0.077 |
|
Patients with dose 7 |
6.711 ± 0.229 |
2.15 ± 0.104 |
|
P-value |
p < 0.001* |
p < 0.001* |
* represents a significant difference at 0.05 level, Enova test.
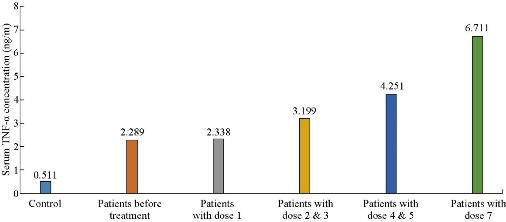
Fig. 2 Association between the dose of treatment and serum TNF-α in control and patient groups.

Fig. 3 Association between the dose of treatment and serum IL-1β in patients groups.
Conclusions
Cytokines (IL-1β and TNF-α) play an essential role in the resolution of CL infection; the antimonial therapy effect on circulating levels proinflammatory cytokines is associated with high levels of IL-1β and TNF-α after 7 days postinfection as compared to that absorbed in pretreatment patients, and this increased production in cytokines may be associated with the killing or control of parasites in the macrophage.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Hasan Raheem Khudhur, Ahmed Abbas Hasan, and Rawaa Adday Ali. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.