Research Article
Preparation and Biological Activities of Some Heterocyclic Compounds Derivatives from 2-Aminothiazoles
Faez Abdul-hussein Alrammahi *
Department of Chemistry, Faculty of Education for Girls, University of Kufa, Al-Najaf, Iraq.
* Corresponding author. E-mail: faez.alrammahi@uokufa.edu.iq
Received: Mar. 12, 2018; Accepted: May 4, 2018; Published: May 8, 2018
Citation: Faez Abdul-hussein Alrammahi, Preparation and Biological Activities of Some Heterocyclic Compounds Derivatives from 2-Aminothiazoles. Nano Biomed. Eng., 2018, 10(2): 129-140.
DOI: 10.5101/nbe.v10i2.p129-140.
Abstract:
In the current investigation, two series of 2-amino thiazole derivatives were prepared. The first series involved synthesis of (Z)-3-(thiazol-2-ylimino)indolin-2-one (A1) as Schiff base derivatives of 2-amino thiazole and isatin, then synthesis of compounds A5, A6 and A7 as five membered rings (imidazolidins) by using different amino acids, and synthesis of compounds A3 and A4 as seven membered rings (1,3-oxazepine-di-one) by using maleic and phthalic anhydrides respectively. In the second series, 2-amino thiazole was treated with acetyl acetone to form (2E,4E)-N2,N4-di(thiazol-2-yl)pentane-2,4-diimine (A2) as di-Schiff base derivatives, and finally the preparation of imidazolidin (A8) by using 2 mol of tyrosine and preparation of tetrazole derivative (A9) by using 2 mol of sodium azide. The biological study for the above two series indicated that both gram-negative and gram-positive activities were noticed.
Keywords: 2-aminothiazoles; Tetrazole; Imidazolodine; Benzoxazepine; Oxazepin; Isatin; Schiff base
Introduction
Thiazoles are five- membered heterocyclic aromatic compound which was first prepared by A. Hantzsch [1] in 1888. The ability of aminothiazoles to form proton-transfer complexes was developed in order to gain structural information enabling a study of the effect of amino group on 2-aminohistamine derivatives [2, 3]. The synthesis of 2-aminothiazoles derivatives has several problems including low yield, harsh reactions, difficult isolation procedures, use of expensive catalysts [4-6] and so on. Thiazole derivatives are significantly important heterocyclic compounds which exhibit a wide range of biological activities such as fungicidal [7], bactericidal [8], cardiovascular [9], antitumor [10], anti-allergic [11], central nervous system stimulate [12, 13] and antipyretic [14]. Due to the broad range of their applications, 2-aminothiazoles have been used in the synthesis of polymers [15, 16]. In this study, some of the heterocyclic compound derivatives from 2-aminothiazoles were prepared and investigated. Scheme 1 summarized pathway of the preparation of compounds A1-A9.
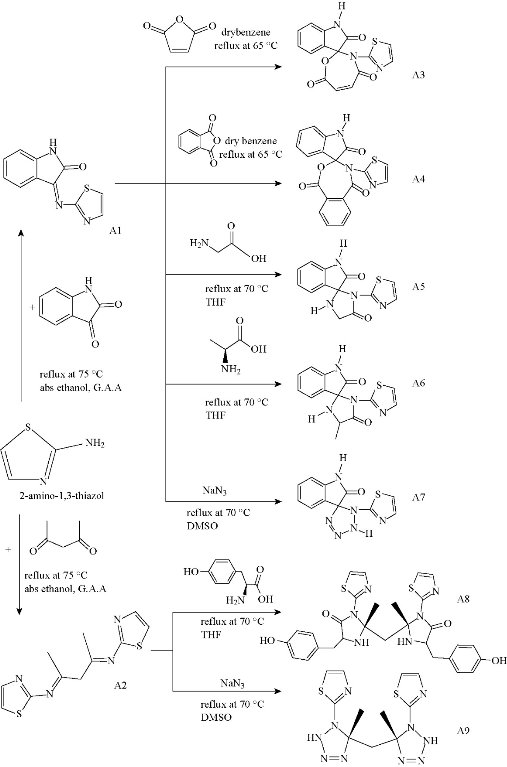
Scheme 1 Synthetic pathway of the preparation of compounds A1-A9
Experimental
Physical properties of compounds A1-A9 are listed in Table 1.
Table 1 Physical properties for product compounds.
|
Compounds |
Molecular formula |
Molecular weight (gm/mol) |
m. p. (℃) |
Colors |
Ylide (%) |
Methanol: Benzene = 1:4 |
|
A1 |
C11H7N3OS |
239 |
195 |
Red |
78 |
0.62 |
|
A2 |
C11H12N4S2 |
264 |
107-108 |
Dark Brawn |
67 |
0.76 |
|
A3 |
C15H9N3O4S |
327 |
175 |
Red Brown |
83 |
0.42 |
|
A4 |
C19H11N3O4S |
377 |
165 |
black Brown |
80 |
0.41 |
|
A5 |
C13H10N4O2S |
166 |
177-179 |
Deep Brown |
74 |
0.53 |
|
A6 |
C14H12N4O2S |
300 |
144 |
Dark Red |
79 |
0.43 |
|
A7 |
C11H8N6OS |
277 |
180-181 |
Brown |
77 |
0.52 |
|
A8 |
C29H30O4N6S2 |
590 |
165 |
Brown |
79 |
0.58 |
|
A9 |
C11H14N10S2 |
350.43 |
174 |
Dark Brawn |
65 |
0.44 |
Synthesis of (Z)-3-(thiazol-2-ylimino)indolin-2-one (A1)
Isatin (6 mmol, 0.6 g) was dissolved in (30 mL) absolute ethanol (abs. EtOH) in a 100 mL round bottom flask; two drops of glacial acetic acid (GAA) were added and stirred; 0.883 g of 6 mmol 2-aminothiazole was added. The reaction mixture was refluxed for 7 h at 70-75 ℃. Progress of the reaction was monitored by thin layer chromatography (TLC); the resulting product was washed, dried and recrystallized from ethanol. Fourier-transform infrared spectroscopy (FTIR / cm: 1637 (υC=N, imine); 1750 (υC=O, carbonyl of indolone); 1450 and 1500 (υC=C, aromatic and vinyl and υC=N, thiazole, vib. coupling); 3450 (N-H, of indolone); 3135 (C-H, aromatic); and 767 (δo.o.p. C-H, benzene) (Fig. 1; Table 2). Proton nuclear magnetic resonance (1H- NMR): ɗ / ppm: 7.32 - 7.951 (s, 4H, phenyl); 7.271 (2H, olifinic of thiazole); and 8,049 (s, H, N-H, indolone) (Fig. 2; Table 3). Elemental analysis calculated for A1 (C11H7N3OS): C, 61; H, 3.16; N, 19.5; S, 14.6; Found C, 61.47; H, 3.28; N, 19.7; and S, 15 (Table 4).
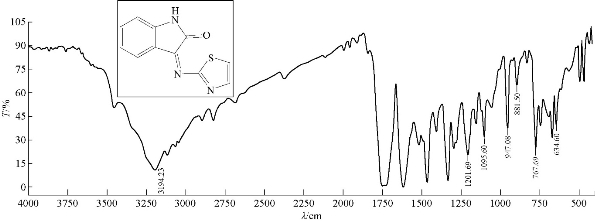
Fig. 1 FTIR spectrum of compound A1.
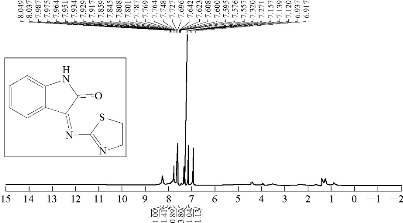
Fig. 2 1H-NMR spectrum of compound A1
Synthesis of (N,N'E,N,N'E)-N,N'-(pentane-2,4-diylidene)bis(thiazol-2-amine) (A2)
0.3 g of 3 mmol acetyl acetone was dissolved in 30 mL abs. EtOH in a 100 mL round bottom flask; three drops of GAA were added and stirred; 0.6 g of 6 mmol 2-aminothiazole was added. The reaction mixture was refluxed for 8 h at 70 ℃. Progress of the reaction was monitored by TLC. The resulting product was washed, dried and recrystllizaed from ethanol. FTIR / cm: 1608 (υC=N, imine); 1589 and 1500 (υC=C, vinyl and υC=N, thiazole, vib. coupling); 3450 (N-H, of indolone); 3182 (C-H, aromatic); 2972 (C-H, methyl); 1442 (C-N, thiazole); and 1041.6 (C-S, thiazole) (Fig. 3; Table 2). H1- NMR: ɗ / ppm: 1.408 (s, 6H, methyl); 6,917-7.271 (2H, olifinic of thiazole); and 2.419 (s, CH2) (Fig. 4; Table 3). Elemental analysis for A1 (C11H7N3OS): C, 61; H, 3.16; N, 19.5; S, 14.6; Found C, 61.47; H, 3.28; N, 19.7; and S, 15 (Table 4).
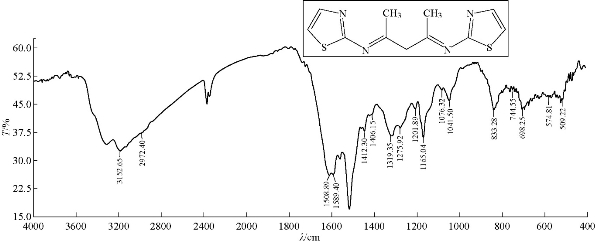
Fig. 3 FTIR spectrum of compound A2.
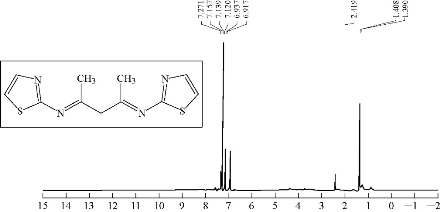
Fig. 4 1H-NMR spectrum of compound A2.
Synthesis of 3'-(thiazol-2-yl)-3'H-spiro[indoline-3,2'-[1,3]oxazepine]-2,4',7'-trione (A3)
A mixture (6 mmol, 0.128 g) of compound A1 and 0.059 g of 6 mmol maleic anhydride were refluxed in 30 mL benzene, at 60 ℃ for about 20 h, and then recrystllizaed from ethanol. FTIR / cm: 1680 (υC=O, amide); 1735 (υC=O, carbonyl of indolone and ester); 1618 and 1462 (υC=C, aromatic and vinyl, vib. coupling); 3400 (N-H, of indolone); 3192 (C-H, aromatic); and 750 (δo.o.p. C-H, benzene) (Fig. 5; Table 2). H1- NMR: ɗ / ppm: 7.584 - 7.484 (s, 4H, phenyl); 7.009-6.577 (2H, olivinic of oxazepine); 6.390 (2H, olivinic of thiazole); and 8,094 (s, H, N–H, indolone) (Fig. 6; Table 3). Elemental analysis for A3 (C15H9N3O4S): C, 54.5; H, 2.5; N, 12.24; S, 14.6; Found C, 55.02; H, 2.72; N, 12.80; and S, 9.71 (Table 4).
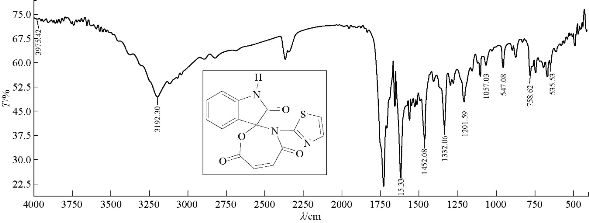
Fig. 5 FTIR spectrum of compound A3.
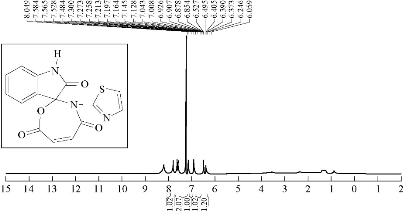
Fig. 6 1H-NMR spectrum of compound A3.
Synthesis of 4-(thiazol-2-yl)-1H-spiro[benzo[e][1,3]oxazepine-3,3'-indoline]-1,2',5(4H)-trione (A4)
A mixture (6 mmol, 0.128 g) of compound A1 and 0.089 g of 6 mmol phthalic anhydride were refluxed in 30 mL benzene, at 75 ![]() for about 24 h, and then recrystllizaed from ethanol. FTIR / cm: 1727 (υC=O, amide); 1651 (υC=O, carbonyl of indolone and ester); 1618 .8 (υC=C, aromatic and vinyl, vib. coupling); 3381 (N-H, of indolone); 3194 (C-H, aromatic); and 669 (δo.o.p. C-H, benzene) (Fig. 7; Table 2). Elemental analysis for A4 (C19H11N3O4S): C, 60.00; H, 2.86; N, 10.98; S, 8.35; Found C, 60.47; H, 2.91; N, 11.14; and S, 8.48 (Table 4).
for about 24 h, and then recrystllizaed from ethanol. FTIR / cm: 1727 (υC=O, amide); 1651 (υC=O, carbonyl of indolone and ester); 1618 .8 (υC=C, aromatic and vinyl, vib. coupling); 3381 (N-H, of indolone); 3194 (C-H, aromatic); and 669 (δo.o.p. C-H, benzene) (Fig. 7; Table 2). Elemental analysis for A4 (C19H11N3O4S): C, 60.00; H, 2.86; N, 10.98; S, 8.35; Found C, 60.47; H, 2.91; N, 11.14; and S, 8.48 (Table 4).
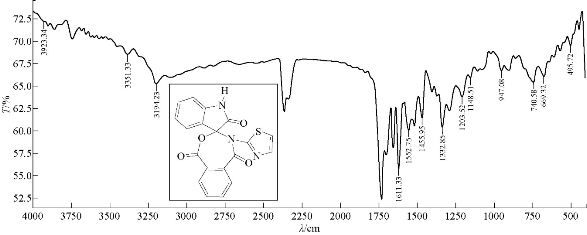
Fig. 7 FTIR Spectrum of compound A4.
Synthesis of 1-(thiazol-2-yl)spiro[imidazolidine-2,3'-indoline]-2',5-dione (A5)
A mixture (0.47 mmol , 0.085 g) of compound A1 and 0.03 g of 0.47 mmol glycine (an amino acid) were refluxed in 30 mL tetrahydrofuran (THF), at 70 ℃ for about 24 h, and then recrystallized from ethanol. FTIR / cm: 1738 (υC=O, amide); 1506 (υC=N, carbonyl); 1620 (υC=C, aromatic and vinyl, vib. coupling); 3292 and 3194 (2N-H, of indolone and imidazolodine); 3052 (C-H, aromatic); 2956 (C-H, aliphatic); and 656 (δo.o.p. C-H, benzene) (Fig. 8; Table 2).
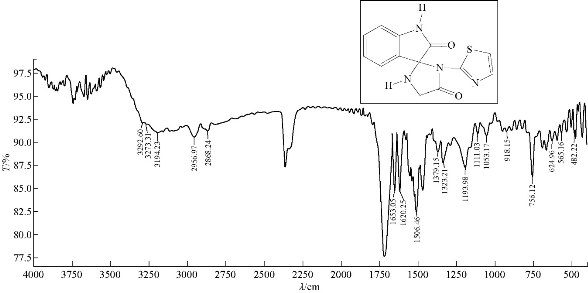
Fig. 8 FTIR spectrum of compound A5.
Synthesis of 4-methyl-1-(thiazol-2-yl)spiro[imidazolidine-2,3'-indoline]-2',5-dione (A6)
A mixture (6 mmol, 0.128 g ) of compound A1 and 0.089 g of 6 mmol alanine (an amino acid) were refluxed in 30 mL THF, at 70 ℃ for about 24 h, and then recrystallized from ethanol. FTIR / cm: 1725 (υC=O); 1651 (υC=O, carbonyl of amide); 1620 (υC=C, aromatic and vinyl); 3300 and 3194 (2N-H, of indolone and imidazolodine); 1512 (νC = N, of thiazole); 3050 (C-H, aromatic); 2928 (C-H, alphatic); and 677 (δo.o.p. C-H, benzene) (Fig. 9; Table 2). Elemental analysis for A6 (C14H12N4O2S): C, 60.00; H, 2.86; N, 10.98; S, 8.35; Found C, 60.47; H, 2.91; N, 11.14; and S, 8.48.
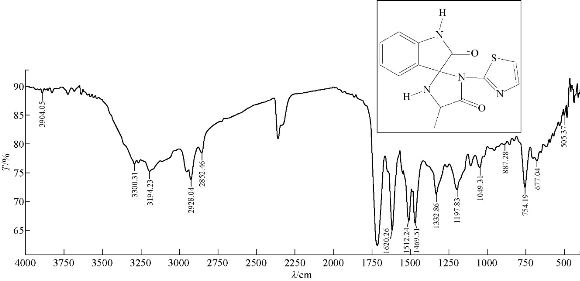
Fig. 9 FTIR spectrum of compound A6.
Synthesis of 1'-(thiazol-2-yl)-1',2'-dihydrospiro[indoline-3,5'-tetrazol]-2-one (A7)
A mixture (4.7 mmol, 0.1 g) of compound A1 and 0.031 g of 4.7 mmol sodium azide were refluxed in 20 mL THF, at 70 ℃ for about 12 h, and then recrystallized from ethanol. FTIR / cm: 3487 and 3250 (2N-H, of tetrazole); 2856 (C-H, aliphatic); 1622 (υC=C, of vinyl in thiazole); 1629 (υC=N, of thiazole); 1508 (υN=N, of tetrazole); and 667 (δo.o.p. C-H, benzene) (Fig. 10; Table 2).
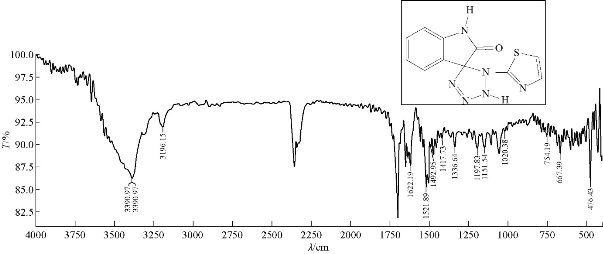
Fig. 10 FTIR spectrum of compound A7.
Synthesis of (2S,2'R)-2,2'-methylenebis(5-(4-hydroxybenzyl)-2-methyl-3-(thiazol-2-yl)imidazolidin-4-one) (A8)
A mixture (1.5 mmol, 0.396 g ) of compound A2 and 0.543 g of 3 mmol tyrosine (an amino acid) were refluxed in 30 mL THF, at 70 ℃ for about 24 h, and then recrystallized from ethanol. FTIR / cm: 1608 (υC=N, imine); 1589 and 1500 (υC=C, vinyl and υC=N, thiazole, vib. coupling); 3450 (N-H, indolone); 3182 (C-H, aromatic); 2972 (C-H, methyl); 1442 (C-N thiazole); and 1041.6 (C-S thiazole) (Fig. 11; Table 2). H1- NMR: ɗ / ppm): 1.408 (s, 6H, methyl); 6,917-7.271 (2H, olifinic of thiazole); and 2.419 (s, CH2) (Fig. 12; Table 3). Elemental analysis for A8 (C29H30O4N6S2): C, 61.09; H, 4.91; N, 14.84; S, 11.33; Found C, 61.45; H, 5.03; N, 15.17; and S, 11.52 (Table 4).
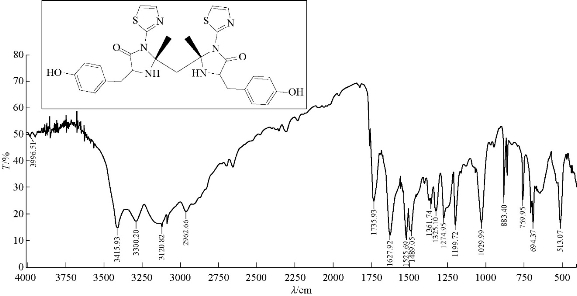
Fig. 11 FTIR spectrum of compound A8.
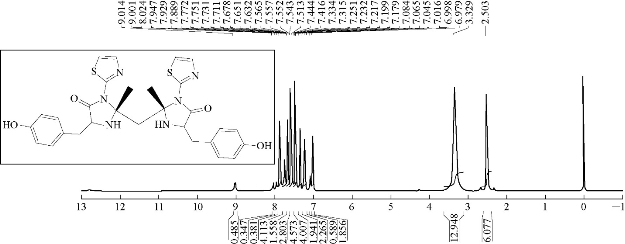
Fig. 12 1H-NMR spectrum of compound A8.
Synthesis of ((R)-5-methyl-1-(thiazol-2-yl)-2,5-dihydro-1H-tetrazol-5-yl)((S)-5-methyl-1-(thiazol-2-yl)-2,5-dihydro-1H-tetrazol-5-yl)methane (A9)
A mixture (1.0 mmol , 0.264 g ) of compound A2 and 0.13 g of 2.0 mmol sodium azide were refluxed in 30 mL dimethyl sulfoxide (DMSO), at 70 ℃ for about 14 h, and then recrystallized from ethanol. FTIR / cm: 3400 and 3363 (N-H, of tetrazole); 2856 (C-H, aliphatic); 1629 (υC=N, of thiazole); and 1592 (υC=N, thiazole) (Fig. 13; Table 2).
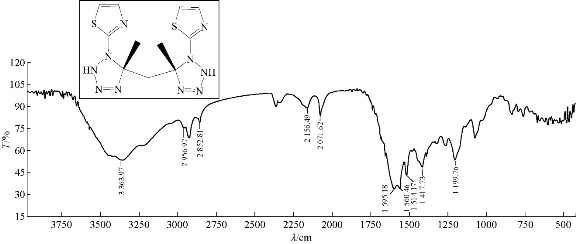
Fig. 13 FTIR spectrum of compound A9.
Table 2 FTIR for product compounds A1-A9
|
FTIR / cm |
Compounds |
||||||
|
Aliphatic υ(C-H) |
Aromatic υ(C-H) |
Aromatic υ(C=C) |
Imine υ(C=N) |
Amide υ(C=O) |
|
||
|
2900 |
3135 |
1450.09 |
1637 |
1750 |
A1 |
||
|
Thiazole υ(C-S) |
Thiazole υ(C-N) |
Aromatic υ(C-H) |
Aliphatic υ(C-H) |
Thiazole υ(C=N) |
Imine υ(C=N) |
|
|
|
1041 |
1442 |
3182 |
2972 |
1589 |
1608.96 |
A2
|
|
|
Amine υ(NH) |
Aliphatic υ(C-H) |
Aromatic υ(C-H) |
Alkene υ(C=C) |
Amide υ(C=O) |
Ester υ(C=O) |
|
|
|
3400 |
2985 |
3194 |
1618, 1462 |
1680 |
1735 |
A3 |
|
|
3381 |
2995 |
3194 |
1618 |
1651 |
1724 |
A4 |
|
|
Hydroxyl υ(OH) |
Thiazole υ(C=N) |
Aromatic υ(C-H) |
Aliphatic υ(C-H) |
Alkene υ(C=C) |
Amine υ(NH) |
Ketone υ(C=O) |
|
|
-- |
1506 |
3052 |
2956 |
1620 |
3194, 3292 |
1738, 1653 |
A5 |
|
-- |
1512 |
3050 |
2928 |
1620 |
3194, 3300 |
1725 |
A6 |
|
3415 |
1525 |
3120.82 |
2962 |
1627 |
3300 |
1735.93 |
A8 |
|
Aliphatic υ(C-H) |
Amine υ(NH) |
Imine υ(C=N) |
Azo υ(N=N) |
Keton υ(C=O) |
|
||
|
2856 |
3250, 3487 |
1629 |
1508.38 |
1745 |
A7 |
||
|
2956 |
3363 |
1592 |
1560 |
-- |
A9 |
||
Table 3 H1-NMR for product compounds A1, A2, A3 and A8
|
Molecular formula. |
OH hydroxyl |
CH aromatic |
CH olivine of oxazepine |
CH olivine of thiazole |
NH indolone |
CH3 methyl |
CH2-phenyl |
|
|
A1 |
C11H7N3OS |
-- |
7.32 - 7.951 |
-- |
7.271 |
8,049 |
-- |
-- |
|
A2 |
C11H12N4S2 |
-- |
-- |
-- |
6,917 - 7.271 |
-- |
1.408 |
2.419 |
|
A3 |
C15H9N3O4S |
-- |
7.584 - 7.484 |
7.009 - 6.577 |
6.390 - 6.244 |
8,094 |
-- |
-- |
|
A8 |
C29H30O4N6S2 |
9.014 |
7.772 - 7.416 |
-- |
7.929 |
7.947 - 7.839 |
2.503 |
3.329 - 7.016 |
Table 4 Elemental analysis for product compounds A1, A3, A4, A6 and A8
|
O% |
S% |
N% |
H% |
C% |
Calculated / Observed |
Molecular formula |
Compounds |
|
6.99 |
14.40 |
19.00 |
3.16 |
58.22 |
Cal. |
C11H7N3OS |
A1 |
|
-- |
14.00 |
18.57 |
3.28 |
57.47 |
Obs. |
||
|
19.55 |
9.20 |
12.24 |
2.50 |
54.53 |
Cal. |
C15H9N3O4S |
A3 |
|
-- |
9.71 |
12.80 |
2.72 |
55.02 |
Obs. |
||
|
16.96 |
8.35 |
10.98 |
2.86 |
60.00 |
Cal. |
C19H11N3O4S |
A4 |
|
-- |
8.48 |
11.14 |
2.91 |
60.47 |
Obs. |
||
|
10.66 |
10.18 |
18.21 |
3.40 |
55.89 |
Calc. |
C14H12N4O2S |
A6 |
|
-- |
10.7 |
18.65 |
3.67 |
56.10 |
Obs. |
||
|
10.83 |
11.33 |
14.84 |
4.91 |
61.09 |
Cal. |
C29H30O4N6S2 |
A8 |
|
-- |
11.52 |
15.17 |
5.03 |
61.45 |
Obs. |
Results and Discussion
Biological activity
According to the World Health Organization (WHO), microorganisms are still the main obstacle to the treatment of infectious diseases, and the preparation of therapeutic agents as a highly selective antimicrobials within the limits of specific concentrations [17. 18]. Five types of pathogenic bacteria were used in the current study, two of them were gram-negative and the other three were gram-positive. The sensitivity was calculated using 1000 ppm degree drilling method, and the employed solvent was DMF [19, 20]. The composition of bacterial cell walls in the gram-negative bacteria presented lower thickness and higher lipophilicity as compared with those of the gram-positive bacteria, thus facilitating the passage of compounds which could be dissolved into the fatty compounds and transferred into the cell. These compounds inhibited the capture. The bacterial growth through the hardiness of the active sites depended on the Overton concept, the phenomenon of plasmolysis, i.e. a retraction of the protoplasm from the cellulose wall of plant cells exposed to impermeable external solutes. (Overton's system explored the relationship between the chemical constitution of solutes, mainly organic, and their plasmolytic effects, with reference to the properties of the putative osmotic barrier) [21-24]. These sites for respiration and protein synthesis inhibit the formation of hydrogen bonds of some interface-active center. Certain cell components or enzymes cause them to stop and change the natural states of cell, leading ultimately to cell death [25]. Table 4 shows the results of biological activity data and the inhibition range in millimeters. Fig. 14 illustrates bacteria wall and the method of using a gram dye to differentiate between gram-positive and gram-negative bacteria. Statistical representation for antibacterial activity of compounds A1-A9 is shown in Fig. 15 and Table 15.
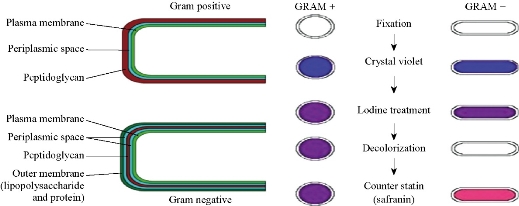
Fig. 14 Bacteria wall and the method of using a gram dyes to differentiate between gram-positive and gram-negative bacteria.
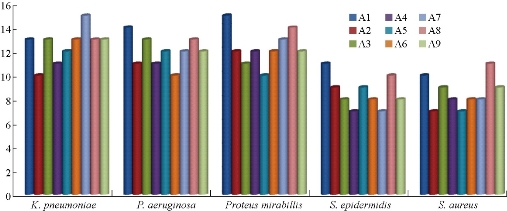
Fig. 15 Statistical representation for antibacterial activity of compounds A1-A9.
Table 5 Biological activity data (zone of inhibition in mm) of the compounds A1-A9.
|
Compounds |
Bacteria |
||||
|
Gram-negative |
Gram-positive |
||||
|
|
K. pneumoniae |
P. aeruginosa |
S. epidermidis |
S. aureus |
|
|
A1 |
13 |
14 |
15 |
11 |
10 |
|
A2 |
10 |
11 |
12 |
9 |
7 |
|
A3 |
13 |
13 |
11 |
8 |
9 |
|
A4 |
11 |
11 |
12 |
7 |
8 |
|
A5 |
12 |
12 |
10 |
9 |
7 |
|
A6 |
13 |
10 |
12 |
8 |
8 |
|
A7 |
15 |
12 |
13 |
7 |
8 |
|
A8 |
13 |
13 |
14 |
10 |
11 |
|
A9 |
13 |
12 |
12 |
8 |
9 |
Conclusions
Two series of 2-amino thiazole derivatives were prepared and characterized in this study. FTIR spectroscopy and NMR technique confirmed the formation of these derivatives. Five types of pathogenic bacteria were used, two of them were gram-negative and the other three were gram-positive. A significant biological activity was observed for the above compounds.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Faez Abdul-hussein Alrammahi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.