Research Article
Anticancer Drug 5-Fluorouracil in Aqueous Solution by Differential Pulse Polarography: An Assessment of Optimum Conditions
Razzaq Abd Al-Zahra Ibrahim 1*, Hussein Kadhem Al-Hakeim 1, Falah Shareef Abed Suhail 2
1 Chemistry Department, Science Faculty, Kufa University, 54001 Najaf, Iraq
2 Pharmaceutical Chemistry Department, Pharmacy Faculty, Kufa University, 54001 Najaf, Iraq
* Corresponding author. E-mail: razzaqalameryi@gmail.com
Received: Mar. 22, 2018; Accepted: Apr. 26, 2018; Published: Apr. 27, 2018
Citation: Razzaq Abd Al-Zahra Ibrahim, Hussein Kadhem Al-Hakeim, and Falah Shareef Abed Suhail, Anticancer Drug 5 - Fluorouracil in Aqueous Solution by Differential Pulse Polarography: An Assessment of Optimum Conditions. Nano Biomed. Eng., 2018, 10(2): 117-128.
DOI: 10.5101/nbe.v10i2.p117-128.
Abstract
The potassium phosphate buffer as a supporting electrolyte of anticancer drug (5-fluorouracil (5-FU)) was the best among solutions of sodium phosphate buffer and Britton Robinson buffer in differential pulse polarography (DPP) at pH = 7.0 and T = 37 oC. The changes of temperature did not effect on inactivity of the supporting electrolyte (potassium phosphate buffer at T = 10 - 50 oC), and pH of the solution did not exceed 2% of each 5 oC (at pH = 7.0, by modified thermostat cell). However, the frequency measurements showed clear effect of temperature on diffusion current (IP /μA) of the chemotherapy compound in the range of 20 - 50 oC and under primary conditions. Then, the polarography measurements of 5-FU drug (at 10 μM, pH = 7 and T = 37 oC) gradually led to the optimum conditions: deposition potential = – 0.9 V; drop size = 9.0 mm3; deposition time = 15.0 sec; equilibration time = 5.0 sec; pulse amplitude = 100 mV; pulse time = 7.0 msec; voltage step = 6 mV; voltage step time = 0.3 sec; and sweep rate = 20.0 mV/sec. The thermal assessment of 5-FU drug (after achievement of the optimum conditions) in a new thermostat vessel at HMDE by DPP showed that the reaction of 5-FU molecules represented pseudo first order reaction, instead of first order); the secondary waves of 5-FU drug may be due to formation of molecular complexes in aqueous solution and the reduction of 5-FU molecules at mercury surface electrode appeared as physisorption, instead of chemisorption.
Keywords: Optimum conditions; Drug 5-fluorouracil (5-FU); Differential pulse polarography (DPP); Behavior of 5-FU
Introduction
In voltammetry, a gradient potential is applied to an electrochemical cell, and the flowed current is measured as a function of that potential. The plot current as a function of the applied potential is called voltammogram which is considered an electrochemical equivalent of spectrum in spectroscopy. It is regarded as the best provider of quantitative (Ip / μA) and qualitative (Ep / V) information about the species involved in oxidation or reduction reaction. The earliest voltammetric technique was polarography, discovered in 1922 by Jaroslav Heyrovsky (Charles University, Prague) who was awarded Nobel Prize in chemistry in 1959 [1]. Polarography is used extensively for analysis of metal ions, inorganic anions and organic compounds that contain reducible or oxidizable functional groups. In general, the pulse polarographic technique represents a suitable pattern for biological fluids analysis in pharmacological investigations. It is available commercially, offers high sensitivity by the comparison with atomic absorption spectroscopy, and is the most chromatographic technique (high-performance liquid chromatography (HPLC) and gas chromatography (GC)) [2]. Fluorouracil is a phase specific drug (5-fluoro-1H-pyrimidine-2,4-Dione) that is a pyrimidine analog used in cancer treatment. It is an antineoplastic drug that interferes with the growth of nucleic acids by substituting it for normal building blocks of RNA and DNA [3]. This agent (trade names: Adrucil, Carac and Efudex) damages the cells during S phase which is present in cell cycle in human body for at least about 18 - 20 h with average 8 - 12 % of protein binding process. Commonly, the anticancer drug (5-fluorouracil (5-FU)) was used to treat leukemia, breast cancers, ovary, and the intestinal tract, as well as other types of cancer (with bioavailability = 28 – 100%). It was designed, synthesized and patented by Charles Heidelberger in 1957, and then was discovered as an inhibitor through irreversible inhibition of thymidylate synthase mechanism. The 5-FU drug is an effective antitumor agent but has many side effects as well as strong toxicity and poor tumor affinity [4, 5]. In this study, assessment of optimum conditions for anticancer drug 5-FU in aqueous solution was studied by differential pulse polarography (DPP).
Experimental
Materials
All chemicals used in the present investigation were obtained from commercial sources (with highest available purity). 5-FU was purchased from Sigma Aldrich (U.S.A.) at 99.99 %. KH2PO4 (potassium dihydrogen orthophosphate), K2HPO4 (dipotassium hydrogen orthophosphate) and Na2HPO4 2H2O (disodium hydrogen orthophosphate dehydrate of phosphate) buffer solution (pH = 5 - 8) were obtained from Sd. Fine Chemical Limited (Mumbai, India) at purity 99.5%. Solution of Britton - Robinson buffer at pH = 7.0 that consisted of Acidic part involving H3BO3 at 99.5%, H3PO4 at 85 %, CH3COOH at 98 % and NaOH as alkaline part at 99.99 %, as well as CH3COONH4 at 99 % (for acetate buffer solution at pH = 4.5). CdCl2 2H2O, Pb(CH3COO)2 3H2O and KCl at purity 99.9 % was from GCC (UK).
Instruments
Computrace polarographic analysis was recorded on a Metrohm type 797VA (Switzerland), version 1.1, 2004 (Fig. 1 and 2) which included two electrodes: rotating disk electrode (RDE); and multi-mode electrode (MME) with three modes, including dropping mercury electrode (DME), static mercury drop electrode (SMDE), and hanging mercury drop electrode (HMDE) which is considered more economic adding to its high sensitivity, and so would be used in the present research as long as was the best. Measurements of pH were adjusted by using Inolap720 pH Meter, WTW (Germany) with Electrode Sentix41. Distilled water produced by GFL apparatus was purified via SG Ionic Exchangers (Germany). The prepared deionized water was measured in Inolap720 Cond Meter, WTW (Germany) with Electrode Tetra Con.325. Temperature of the prepared solutions was regulated by using Julabo Circulator Water Bath (Germany) within 37 oC. All weights were taken by a Mettler Toledo AB 204S, Sensitive electronic balance (Switzerland).
Methods
Buffers and other analyte solutions were prepared according to standard literatures, and measured by calibrated pH meter continuously [6-9]. The modified thermostat cell made manually consisted of circular water bath joined with a polarographic cell by rubber tubes to contact the cell wall with water. For control on the effusion of water, triple divider was put in the outlet vent of circular water bath. The one nick of divider returned part from water to water bath, and the other nick went toward polarographic cell. The arriving water and polarographic cell together were collected in plastic vessel containing water pump which acted on return water into the circular water bath continuously (Fig, 1). The idea of modified thermostat cell may be simple, but it was very important during measurement to keep the temperature constant. Then, new thermostat vessel was designed manually too (Fig. 2). It was less complicated than modified thermostat cell, but the two thermostat units had the same efficiency when the same test was applied in polarographic analyzer; 797VA Computrace. The standardization of polarographic analyzer was achieved by cadmium chloride solution (0.001 M) dissolved in solution of potassium chloride (0.1 M) as supporting electrolyte. The differential pulse polargram included one wave with potential (EP) equal to – 0.603 V as shown in Fig, 3(a). To make guaranteed certification of polarograph in accuracy separation for more than one components, the polarographic measurements were achieved for cadmium and lead solutions (0.001 M) individually (Fig. 3(b) and (c)), and then for mixture of two ions (0.0005 M) in acetate buffer at pH = 4.5 as supporting electrolytes (Fig. 3(d)). The differential pulse polargram included two waves only with peak potentials (EP/V) equal to – 0.564 for Cd2+ and – 0.389 V for Pb2+, respectively. The results of all solutions were identical exactly for standard values cited in literature review [6, 11, 12]. This convention procedure is useful in appearance of the gauge of sensitivity, accuracy and precision of analysis by the polarographic analysis technique. Firstly, solutions of KCl and acetate buffer as supporting electrolytes of ions were measured without any polargram which is inactive electrochemically.

Fig. 1 The modified thermostat cell in polarographic analyzer, 797VA Computrace.

Fig. 2 The new thermostat vessel with a polarographic analyzer, 797 VA Computrace.
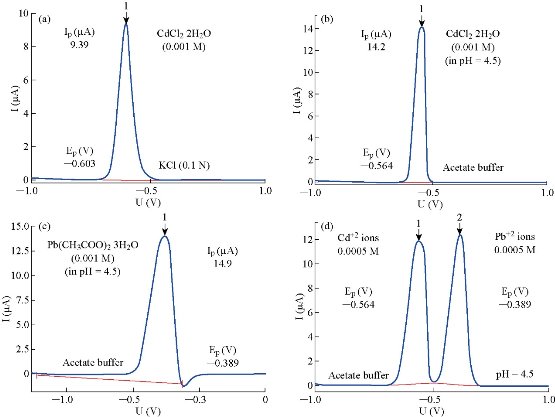
Fig. 3 Standardization of polarographic analyzer, 797 VAcomputrace. (a) 0.001 M CdCl2 2H2O in 0.1 M KCl; (b) 0.001 M CdCl2 2H2O in acetate buffer at pH = 4.5; (c) 0.001 M Pb(CH3COO)2 3H2O in acetate buffer at pH = 4.5; and (d) 0.0005 M Cd2+ and Pb2+ in acetate buffer at pH = 4.5.
Results and Discussion
The anticancer drug 5 FU is distinguished by electrochemical activity, since it has electro active functional groups that can be reducible or oxidizable easily in different voltammetric methods [12]. According to this ideology, 5 FU drug can be followed electrically depending on produced wave by polarographic analysis technique (in DPP at HMDE).
Effect of supporting electrolyte
Before corroboration the optimization instrumental conditions of the polarograph technique, it is very necessary to select a suitable supporting electrolyte of 5 FU drug. The polarographic measurements of different supporting electrolytes were achieved at primary conditions (Table 3), to decide which one the best of anticancer drug. The sodium phosphate buffer was not useful in polarography. It had electrochemical activity represented by waves with high current, while the solutions of potassium phosphate buffer and Britton-Robinson buffer were suitable selection of 5-FU (Fig. 4). The differential pulse polargram of anticancer drug (5-FU) with 10 μM was carried out in Britton – Robinson buffer and potassium phosphate solutions at pH = 7.0; T = 37 oC. The obtained results were shown that Britton Robinson buffer is not suitable, as result to the splitting of sort wave of 5-FU (Fig. 5). Therefore, potassium phosphate buffer solution would be dependent in the subsequent polarographic measurements of 5-FU drug.
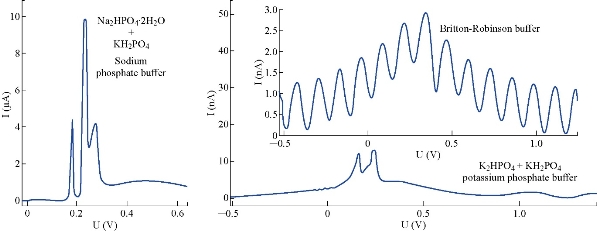
Fig. 4 Polargrams of supporting electrolytes at pH = 7.0 and T = 37 oC by DPP at HMDE.
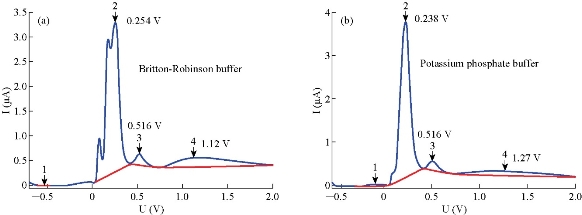
Fig. 5 Polargrams of 5-FU drug (10 μM) (a) Britton-Robinson buffer solution, and (b) potassium phosphate buffer solution, at pH = 7.0 and T = 37 oC.
Effect of temperature [1, 13]
After the active system was bound with cell of polarograph to keep the temperature constant (Fig. 1), the effect of temperature change was examined on supporting electrolyte firstly. This step is very important for determination of the resistance of supporting electrolyte (buffer capacity) at different temperatures. The obtained results showed unaffected polarographic wave of supporting electrolyte dynamically, when the temperature changed within the range of 10 - 50 o C. At the same time, acidity function of phosphate buffer solution with the change of temperature was measured (Table 1), and pH change did not exceed 2% (at pH = 7.0; by modified thermostat cell). Then, the current (IP) of 5-FU drug at 10 μM, pH = 7.0 and in the temperature range 20 - 50 oC increased with temperature directly at primary condition.
Table 1 Effect of temperature on 5-FU wave (Ip, Ep) with 10 μM and the acidity function of phosphate buffer solution (Ph.B) at pH = 7.0
|
T (℃) |
Ep1 (V) |
Ip3 (µA) |
Ip2 (µA) |
Ip1 (µA) |
Ep3 (V) |
Ep2 (V) |
pH of Ph.B |
|
10 |
-- |
-- |
-- |
-- |
-- |
-- |
7.05 |
|
15 |
-- |
-- |
-- |
-- |
-- |
-- |
7.03 |
|
20 |
0.0974 |
0.197 |
5.54 |
1.57 |
0.492 |
0.230 |
7.00 |
|
25 |
0.0194 |
0.223 |
6.07 |
1.67 |
0.5 |
0.222 |
7.00 |
|
30 |
0.0589 |
0.224 |
6.38 |
1.82 |
0.5 |
0.222 |
7.01 |
|
35 |
0.0987 |
0.258 |
7.33 |
1.82 |
0.5 |
0.222 |
7.02 |
|
37* |
0.0990 |
0.263 |
8.06 |
1.77 |
0.5 |
0.222 |
7.02 |
|
40 |
0.111 |
0.273 |
8.95 |
1.82 |
0.5 |
0.214 |
7.03 |
|
45 |
0.114 |
0.298 |
9.07 |
1.76 |
0.5 |
0.214 |
7.08 |
|
50 |
0.114 |
0.284 |
9.76 |
1.75 |
0.5 |
0.206 |
7.12 |
Behavior of anticancer drug 5-FU
The measurement of 5-FU solution at 20 oC did not occur easily, and this result was obtained after many trials with irregular polarographic waves (especially for sort wave). In spite of frequent attempts, the polarographic waves of 5-FU at 15 oC or less did not appear regular. This may be explained by either the inability of analyte molecules to reach the surface electrode (HMDE) or because of the insolubility of 5-FU drug in aqueous solution at T ≤ 15 oC.
Determination of optimum conditions for 5-FU in DPP
The previous study represented a preliminary investigation of 5-FU drug, before the validation on optimum conditions (such as pH and the instrumental parameters). Therefore, initial studies would be directed toward perfection of experimental conditions to establish the optimum conditions which are considered very necessary for the maximum sensitivity in polarography.
Effect of pretreatment parameters
A new stock solution of 5-FU drug (10 μM) was prepared in phosphate buffer solution at pH = 7.0 and 37 oC, to study the effect of pretreatment parameters, including mercury drop size, deposition potential, deposition time and equilibration time. It is very important to check these parameters in HMDE of polarography, which is considered more economic in mercury and highly sensitive in comparison to other modes. In general, the analysis process in HMDE is distinguished by three main steps: First, the pre-electrolysis involved accumulation of analyte molecules under constant potential on the working electrode; this step is recognized by deposition time. The second step involves leaving the analyte solution stable; it is acknowledged by equilibrium time. The third step includes the reversible analysis, i.e. removing concentrated molecules of analyte about the surface of working electrode by applying contrast potential for that used in the first step. Therefore, HMDE mode in a polarographic technique was called stripping voltammetry [11, 14]. The effect of drop size (D.S) on wave current of 5-FU new stock solution (fresh perpetrated with 10 μM in phosphate buffer solution at pH = 7.0 and 37 oC) was studied in the range of 1 - 9 mm3. The obtained results (Fig. 6) showed that D.S at 9 mm3 represented the best mercury drop size, giving the highest diffusion current; hence, it would be considered the ideal drop size of 5-FU drug in subsequent studies. The deposition potential (D.P) as the representation of the applied potential at deposition time step on electrode (HMDE) in stripping voltammetry was studied at different negative and positive values (under the same conditions). However, the deposition potential (within the range of – 0.9, 0, + 0.9 V) had no effect on current wave of 5-FU; this means that 5-FU had the net charge which may be equal or approach to zero. The obtained result represent the same effect occurring after oxidation of 5- FU by using the glassy carbon electrode (GCE) in differential pulse and cyclic voltammetry [15]. This was represented as an expect behavior of 5-FU in aqueous solution by using voltammetry techniques, which means the independence of 5-FU wave on charge of electrode surface. According to this, 5-FU drug has similar performance in reduction by HDME or oxidation at GCE. Therefore, the deposition potential at – 0.9 V would be dependable in the next polarographic measurements. There was simple probability that the incompletion of optimum conditions might be affected on and any difference would be observed. So, after the optimization, the effect of deposition potential on current would be repeated in order to ensure this result. The change of deposition time (D.T) within the period of 0 - 80 sec was examined for 5-FU solution at 10 μM, pH = 7.0 and 37 oC. The deposition time change did not bring any significant transformation in a wave potential, but the wave current increased gradually with the increase of deposition time. Generally, the deposition time was proportional with the wave current (Ip) directly at D.T = 5 - 15 sec for a sort wave of 5-FU only. The optimum deposition time had a largest current of sort wave, and the changed regularity was 15 sec which would thus be considered as a selected value for optimization. The polarographic measurements were carried out for 5-FU solution at 10 μM, pH = 7.0 and 37 oC, to select optimal equilibrium time (E.T). The effect of the parameter E.T was studied in the range of 0 - 75 sec at constant other conditions (D.S = 9 mm3, D.P = – 0.9 V and D.T = 15 sec). The wave that had optimal properties (as a higher value of current, constant potential and symmetrical shape) was at E.T = 5 sec, which would thus be relied on in next measurements of 5-FU drug. So, the current (Ip / μA) and the potential (Ep / V) of 5-FU wave at optimum conditions could be summarized in Table 2.
Table 2 Optimum pretreatment conditions of 5-FU (10 μA, pH = 7.0 and 37 oC) in HMDE by DPP
|
No. |
Optimum condition |
Ep1 (V) |
Ep2 (V) |
Ep3 (V) |
Ip1 (µA) |
Ip2 (µA) |
Ip3 (µA) |
|
(1). |
D.S = 9 mm3 |
0.222 |
0.508 |
1.75 |
10.3 |
0.308 |
0.173 |
|
(2). |
D.P = – 0.9 V |
0.222 |
0.508 |
1.75 |
10.3 |
0.308 |
0.173 |
|
(3). |
D.T = 15 sec. |
0.222 |
0.508 |
1.75 |
10.7 |
0.316 |
0.171 |
|
(4). |
E.T = 5 sec. |
0.222 |
0.508 |
1.75 |
11.2 |
0.297 |
0.167 |
Effect of hydrodynamic parameters
The effect of hydrodynamic parameters, represented by pulse amplitude, pulse time, voltage step and voltage step time, were studied for the same stock solution of 5-FU at 10 μM, pH = 7.0, T = 37 oC and constant pretreatment parameters. It is very necessary to assess these parameters of DPP mode, because they have direct effect on symmetry and regularity of 5-FU wave (Fig.7). In this level, the new thermostat vessel (Fig. 2) was used instead of the modified thermostat cell (Fig. 1) that was useful in the above steps (with a same efficiency of polarographic measurements). The effect of pulse amplitude (P.A) of 5-FU drug was studied in the range of 10 - 300 mV. It was found that the wave current (Ip1) increased directly with the progress of pulse amplitude, but the changes of wave potential (Ep1) were not homogenous even at the sort wave. The same measurements appeared asymmetric in shape of sort wave especially, and the second wave became deformed clearly in the range of 125 - 300 mV which appeared as a maxima inside sort wave calculated by wave evaluation icon. It was noticed that the line plan of waves during measurement was sharper and more micro at 10 - 35 mV, and after these values the regularity began gradually (in zigzag form) reaching for the ideality at 100 mV as shown in Fig. 8. Mathematically, area under the peak and charge of second wave does not appear at P.A = 100 mV; it becomes zero with keeping the third wave shape in all measurements at the same state. Therefore, the pulse amplitude had high performance of symmetric wave shape and large height wave relatively, and the wave potential acceptable will represent the optimal value. Hence, 100 mV as a pulse amplitude was the preferred choice containing all those properties in this work. The effect of pulse time (P.T) on wave current and potential of 5-FU was studied at 1.0 - 200 msec. At the beginning of measurement (with P.T = 1.0 – 5.0 msec), a warning appeared as “the lowest selected current range is too low with respect to the sweep rate”. But when pressing OK in the open listing, the measurement was continuous normally to give positive results. The second wave disappeared at P.T = 1 - 10 msec; then it became visible at 20 msec, and the values were calculated by wave evaluation icon or by peak search icon. The values at P.T = 60 - 155 msec would be excluded as result of the unclear shape of sort wave which underwent splitting, irregularity and sharpness. Then, the first four measurements (1.0 - 5.0 msec) could not be selected in spite of the high current, because of the maxima inside sort wave and the warranty term. It was a limit of comparison between two values of pulse time 6.0 msec (which had asymmetric wave) and 7.0 msec selected as the optimum value, since it had the best properties (cited in Fig. 9). Also, a new warning term appeared at P.T = 200 msec, and pressing on OK (in the open list) did not made the measurement achieved as occurring previously. The effect of voltage step (V.S) on 5-FU waves (Ip and Ep) was studied in the range of 2.0 - 100 mV. For the first two measurements (2.0 - 4.0 mV), the sweep rate was slowly led to micro plane lines and the current low comparison with primary value. Then, sweep rate became more suitable at V.S = 6 mV; but area under the peak and charge of second wave was equal to zero. At 7.0 - 100 mV, the second wave was converted as maxima inside the sort wave exclusive of 9.0 mV which was calculated by wave evaluation icon and had asymmetric sort wave (flatting). In addition, sweep rate became speedier at V.S ≥ 18 mV, the matter which affected on the shape of waves (irregular) especially at 20 - 100 mV were sharper. Mainly, competition was limited between primary values 7.0, 8.0 and 6.0 mV that had characteristic factors such as suitable sweep rate, symmetric wave more than others (as zigzag form) and the relatively high current of sort wave. Therefore, voltage step at 6.0 mV was considered the preferred choice of 5-FU drug, because it contained all those properties (Fig.10). The voltage step time (V.S.T) was studied within the range of 0.02 - 1.0 sec on 5-FU waves. At 0.02 - 0.025 sec, the alert appeared as “voltage step time cannot be smaller than 0.025S for current range 10 nA”. But when pressing OK in open listing, the measurement was not achieved. Sweep rate at 0.03 - 0.15 sec increased gradually, and the moderating began at the primary value of 0.2 sec. In addition, the second wave in some values disappeared as calculated by wave evaluation or by peak search; area under the peak and charge was equal to zero. At 0.35 - 1.0 sec, sweep rate decreased gradually, approaching the very low sweep at last. The subject of this report was to focus on three values only: V.S.T = 0.20, 0.25 and 0.3 sec. They showed acceptable sweep rate (20 mV/sec) and a zigzag form of wave line that was more suitable than other values. Thus, value of V.S.T = 0.3 sec would be considered the optimum voltage step time of 5-FU drug, although it had a current less than the wave at 0.03 sec (Ip1 = 64.0 μA) as shown in Fig. 11. Before confirmation, optimum conditions must be rechecked for the effect of deposition potential change on 5-FU wave, as mentioned previously in pretreatment parameters. The positive values within the range of 0.9 - 0.0 V appeared asymmetric at the top of sort wave except the one at 0.0 V (Ip = 64.4 µA) which was similar to waves at negative values in symmetry. The waves of 5-FU at different deposition potentials varied in current slightly, but properties of the waves at D.P = – 0.9 V were better than the others; plane lines were regular, micro and more zigzag. Therefore, the value of D.P = – 0.9 V (Ip1 = 58.1 µA) would be considered as optimum value, although some values, such as 0.0 and – 0.2 V had larger current since it failed in frequency measurements. The effect of these conditions on 5-FU wave can be observed in Fig. 5(b) and 11. Then, the simple comparison between primary and optimum conditions can be achieved. Table 3 concludes the difference of the required target (optimum wave). Although difference between the two values of primary and optimum conditions was simple, there was a clear effect on the real diffusion of 5-FU molecules from bulk solution to electrode surface. So, the current of 5-FU at optimum conditions for sort wave especially (Ip1 = 58.1 µA) became more than the same wave at primary conditions (Ip1 = 7.23 µA) at pH = 7.0 and T = 37 oC. Therefore, the complete optimum conditions of 5-FU drug led to increasing the diffusion current by nearly six times higher than the primary value (at primary conditions).

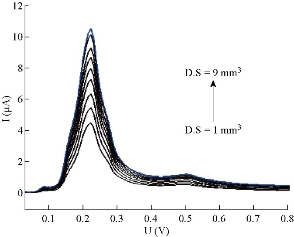
Fig. 6 The mercury drop size in DPP by HMDE of 5-FU drug at 10 μM, pH = 7.0 and T = 37 oC.
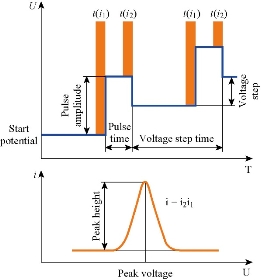
Fig. 7 Differential pulse polargram and effective sweep parameters [11, 12].
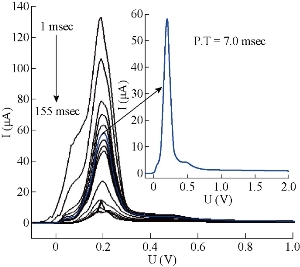
Fig, 8 Wave shapes of 5-FU drug at different pulse amplitude at 10 μM, pH = 7 and T = 37 oC.
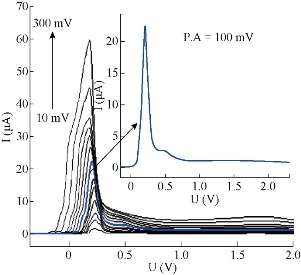
Fig. 9 Wave shapes of 5-FU drug at different pulse time at 10 μM, pH = 7 and T = 37 oC.
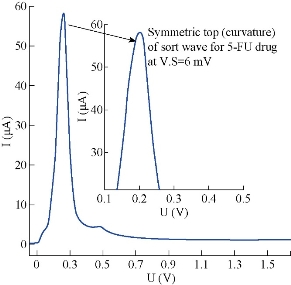
Fig.10 Polarographic wave of 5-FU (10 μM) at voltage step = 6 mV in pH = 7 and T = 37o C.
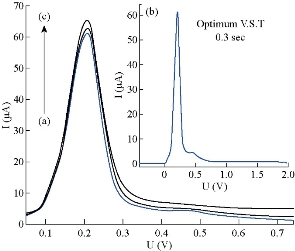
Fig. 11 Polarographic waves of 5-FU (10 μM) at different V.S.T (a). 0.2, (b). 0.3,(c). 0.03 sec.
Table 3 Assessment of optimum conditions for 5-FU drug (10 μM) in the aqueous solution of phosphate buffer at pH = 7.0 and 37 oC by HMDE in DPP.
|
No. |
Instrumental conditions |
(1). Primary conditions |
(2). Optimum conditions |
Δ = 2 – 1 |
Ip1 / μA |
|
|
Primary |
Optimum |
|||||
|
(1). |
I.P.T / sec |
300 |
300 |
0.00 |
-- |
-- |
|
(2). |
D.S / mm3 |
4.0 |
9.0 |
+ 5.00 |
7.23 |
10.3 |
|
(3). |
D.P / V |
– 0.9 |
– 0.9 |
0.00 |
10.3 |
58.1 |
|
(4). |
D.T / sec |
10.0 |
15.0 |
+ 5.00 |
10.3 |
10.7 |
|
(5). |
E.T / sec |
6.0 |
5.0 |
– 1.00 |
10.7 |
11.2 |
|
(6). |
P.A / mV |
50.0 |
100 |
+ 50.0 |
11.2 |
25.0 |
|
(7). |
P.T / msec |
20.0 |
7.0 |
– 13.0 |
25.2 |
57.9 |
|
(8). |
V.S / mV |
8.0 |
6.0 |
– 2.00 |
58.1 |
58.0 |
|
(9). |
V.S.T / sec |
0.2 |
0.3 |
+ 0.10 |
58.0 |
58.1 |
|
(10). |
S.R (mV/sec) |
40.0 |
20.0 |
– 20.0 |
-- |
-- |
Note: I.P.T. = Initial purge time of nitrogen gas; S.R = Sweep rate
Effect of temperature on 5-FU waves (after optimization)
After achievement of optimum conditions, the study of temperature change on waves of 5-FU drug could be repeated, as the real effect of temperature on 5-FU waves did not appear clearly (Table 1) for the above reasons [1, 13]. A polarographic measurement was achieved for 5-FU solution (at 10 μM and pH = 7) within the range of 5 - 100 oC for all five centigrade degrees. The results showed a gradual change of 5-FU current with the temperature directly (Fig. 12), and all the measurements occurred without any difficulty until 75 oC. Then, two secondary waves became deformed. But the regularity of the sort polarographic wave stayed especially. This wave represented the essential wave of 5-FU drug. In addition, the third wave at 50 oC did not appear and at 90 oC was combined together with the second wave to become one broad wave, which in some cases (such at 60 oC) was the area under the peak and charge approached to zero. The sort wave current increased directly with temperature regularly, meaning that 5-FU molecules were moved easily from a bulk solution to electrode surface, without damage or change of molecular structure until at 100 oC. This may result from either increasing the kinetic energy of molecules, or less retardants found as the molecules travelled to electrode, or both reasons. According to these concepts, the shape and current of secondary waves (Ip2, Ip3) changed irregularly, while a sort wave showed regularity and symmetry until at high temperature. This means that the secondary waves might be due to molecular association of 5-FU molecules presented in the solution under study (at 10 μM and pH = 7). Consistent with the supposition, not only the formula of 5-FU molecules was reduced at electrode surface, but the sort wave is represent chief of drug. The assumption is in agreement with the results of Mirceski et al. suggesting the described mechanism (Eqn. 1) of 5-FU reaction at HMDE by square wave voltammetry (SWV). The reduction of 5-FU drug was considered as a chemisorption reaction on electrode surface and a creation of sparingly soluble compound with the electrode material (at pH = 6.7and 0.1M of Na2SO4). Therefore, the redox reaction is established according to first order reaction, i.e. the limited slow step in mechanism depends on 5-FU molecule as follows:
L2 (aq) ⇌ L2 (ads) + Hg ( l ) ⇌ Hg L (s) + 2e (1),
where L2 is the symbol of 5-FU drug as a reacting ligand [16]. Therefore, some conclusions that contribute to understanding mechanism of 5-FU reaction on mercury electrode (HMDE) can be recorded. The reaction of 5-FU molecules on electrode surface (HMDE by DPP) represents a pseudo first order reaction, instead of first order as explained by Mirceski et al. As a result, 5-FU contributed not only a formula in mechanism, but one formula from these species representing significance in limited slow step. The two secondary waves of 5-FU molecules may be especially due to formation of molecular complexes in aqueous solution (at 10 μM and pH = 7), because the analysis of 5-FU drug had more than one functional group such as carbonyl, amide, fluoride group and double bond. Therefore, the creation of molecular complexes have a donor-accepter property which is more possible in aqueous solution according to Dewar-Lepely theory [17, 18]. The evidence of formation of transient molecular complexes was the two distorted secondary waves at high temperature which had low current and inconstant potential. Generally, similar phenomenon of multi waves was observed in cathodic stripping voltammetry of cysteine and other active compounds [16]. The equation that binds the diffusion current of sort wave of 5-FU with temperature was Ip1 = 1.6142 T, and the slope represents a joined relation between the two parameters (Ip1, T) equal to 1.6142 µA / 5 oC. It indicated the increase of temperature by 5 oC led to increase of the diffusion current of 5-FU with the average value equal to 1.6 µA of sort wave. Therefore, the sort wave of 5-FU would be considered the chief wave used in the follow-up reaction of 5-FU molecules, since it had the standard polarographic wave. The assumption was to consider 5-FU reduction (with 50 μM) at HMDE by SWV as a chemisorption reaction on electrode surface at pH = 6.7 and about T = 22 oC [16]. But Fig. 12 shows a reduction of 5-FU with 10 μM at HMDE by DPP as physisorption reaction on surface electrode at pH = 7, because the increase of diffusion current (Ip1) was corresponding with increase of temperature. It means that adsorption of 5-FU molecules on electrode surface may form more than one layer with electrode material (HMDE) [1, 12, 13].
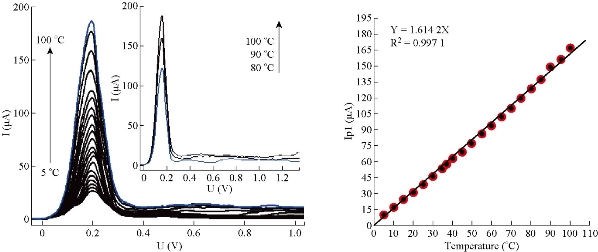
Fig. 12 Polarographic waves of 5-FU drug (10 μM) at different temperatures in aqueous solutions of potassium phosphate buffer at pH = 7.0 and under optimum conditions.
Conclusions
The modified thermostat cell and new thermostat vessel joint with polarographic analyzer (797 VA Computrace) had the same efficiency. The results showed that potassium phosphate buffer was better than sodium phosphate buffer and Britton-Robinson buffer as a supporting electrolyte of 5–FU drug. The rechecked pretreatment and hydrodynamic parameters of 5–FU drug led to increasing the diffusion current by nearly six times higher than the primary value at HMDE by DPP. Effect of temperature showed that 5-FU drug was not only a formula as molecular complexes, but one from these species representing significance in limited slow step. Reduction of 5-FU at HMDE by DPP was physisorption reaction on surface electrode at pH = 7, inverse to the assumption of Mirceski et al. who considered it as a chemisorption reaction at HMDE by SWV.
Conflict of Interests
The authors declare that no competing interest exists.
References
1. D. Harvey, Modern analyitical chemistry. Mc Graw Hill Higher Education, 2000: 508-530.
2. T. Rily, A. Watson, Polarography and other voltammetric methods. John Wily & Sons, 1987: 30-47.
3. American Cancer Society, Chemotherapy principles. An indepth discussion of the techniques and its role in cancer treatment, 2013: 120-129.
4. American Cancer Society, World ovarian cancer day, article paper, 8th. May 2015. CA Cancer J. Clin., 1976, 17: 291-293.
5. S. Ozaki, Synthesis and antitumor activity of 5-fluorouracil derivatives. J. Medicinal Research Reviews, 1996, 16: 51-86.
6. A.I. Vogel, Quantitative in organic analysis, 3rd. ed. Longman Group Limited, 1964: 55-156.
7. D.D. Perrin, B.B. Dempsy, Buffers for PH and metal Ion control. John Wiley and Sons, 1974: 50-86.
8. P.L. Williams, R.C. James, and S.M. Roberts, Principles of toxicology: environmental and industrial application, 2nd. ed. John Wiley & Sons, 2000: 341-251.
9. N. El-Enany, A. El-Brashy, and F. Belal, Polarographic analysis of quetiapine in pharmaceuticals. J. Portugaliae Electrochimica Acta, 2009, 27: 113-125.
10. R. E. Schirmer, Modern methods of pharmaceutical analysis, 2nd. ed. CRC Ltd. Press, 1991: 89-109.
11. Company of Metrohm Ltd., Hardware manual for 797 VA computrace, Switzerland, 1st.ed. Company of Metrohm Ltd., 2004: 10-25.
12. K. Zutshi, Introduction to polarography and allied techniques. 2nd. ed. New Age International Ltd. Press, 2006: 14-28, 58-64, 98-104, 153.
13. M.B. Mohamed, N.T. Adbel-Ghani, O.M. El-Borady et al., 5-fluorouracil induces plasmonic coupling in gold nanospheres: New generation of chemotherapeutic agents. J. Nanomedicine and Nanotechnology, 2012, 3: 1-7.
14. W.C. Pardy, Analytical chemistry method and application. Mc. Graw- Hill, 1989: 89-102.
15. S.R. Sataraddi, S.T. Nandibewoor, Voltammetric oxidation and determination of 5-FU and its analysis in pharmaceuticals and biological fluids at glassy carbon electrode mediated by surfactant cetyltrimethyl ammonium bromide. J. Der Pharma Chemica, 2011, 3: 253-265.
16. V. Mirceski, R. Gulaboski, B. Jordanoski, et al., Square wave voltammetry of 5-fluorouracil. J. of Electroanalytical Chem., 2000, 490: 37-47.
17. J.S. Dewar, R. Lepely, π -complexes. I. Charge transfer spectra of π -complexes formed by trinitrobenzene with polycylic aromatic compounds. J.A.C.S., 1961, 83: 4560.
18. O.A. Hatem, F.S.A. Suhail, and A.M. Juda, Computational and polarographic study on drug-receptor interaction for atenolol. International Journal of Pharmaceutical Sciences Review and Research, 2016, 38(1): 263-270.
Copyright© Razzaq Abd Al-Zahra Ibrahim, Hussein Kadhem Al-Hakeim, and Falah Shareef Abed Suhail. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.