Research Article
Porous Media for Removal of Organic and Inorganic Contaminants
Emad Abbas Jaffar Al-Mulla 1, Fayq Hsan Jabbar2, Rawaa Fahim Chyad AL-Hamadani 3
1 Department Pathological Analysis, College of Health and Medical Techniques, Al-Furat Al-Awsat Technical University, 54003 Al-Kufa, Iraq.
2 Al-Karkh University of Science, 10003 Baghdad, Iraq.
3 Department of Environmental Engineering, Al-Israa University, 10001 Baghdad, Iraq.
* Corresponding author. E-mail: almullaemad@gmail.com
Received: Feb. 24, 2018; Accepted: Apr. 17, 2018; Published: Apr. 25, 2018
Citation: Emad Abbas Jaffar Al-Mulla, Fayq Hsan Jabbar, and Rawaa Fahim Chyad AL-Hamadani, Porous Media for Removal of Organic and Inorganic Contaminants. Nano Biomed. Eng., 2018, 10(2): 104-116.
DOI: 10.5101/nbe.v10i2.p104-116.
Abstract
Wastewater contains heavy metals that cause serious environmental problems. Hence, in the environmental studies, it is important to know the adsorption process of the contaminants in porous media. This study used a continuous flow system which consisted of horizontal PVC pipes of 150 mm diameter and 6 m length. The system was designed and installed in the Environmental Hydraulic Laboratories of Al-Mustansiryiah University (College of Engineering, Environmental Department). This pipe included a manufacturing porous media, activated ceramic (ACR). The objective of this work is to study the adsorption of the total organic carbon (TOC) and the heavy metals from aqueous solutions using manufacturing porous media through horizontal flow without membrane. The discharge of the polluted water to the pipes was changed at values of 10, 20, 40, 60, 80 and 100 L/h. The results of this system were: the removal efficiency of the heavy metals increased with an increase in detention time, length of the pipe and the pressure.
Keywords: Heavy metals; Total organic carbon; Adsorption; Activated ceramic
Introduction
Contamination of aqueous environments by heavy metals is a worldwide environmental problem due to their toxic effects and accumulation through the food chain [2]. The major pollutants are heavy metals in ground, marine, industrial, and even treated wastewaters [3]. The presence of heavy metals in drinking water will be hazardous to consumers. Zn, Cd, Hg, Pb, Cr, Cu, and so forth can damage liver and nerves and block functional groups of vital enzymes and bones [4]. Metal ions in water can occur naturally from anthropogenic sources and from leaching of ore deposits, which mainly include solid waste disposal and industrial effluents. The levels of heavy metals in water system have substantially increased over time with rapid development of industrial activities [5] High zinc can cause eminent health problems, such as stomach cramps, vomiting, skin irritations, anemia, and nausea [6-8]. Various methods are available to remove and isolate these heavy metals from water and wastewater such as ion-exchange, chemical precipitation, membrane filtration, adsorption, and electrochemical treatment technologies [9]. Adsorption is one of the safest, easiest, and most cost-effective methods because it is widely used in effluent treatment processes [10]. On the other hand, solid waste is offered in giant quantities or bound waste merchandise from agricultural operations. Other than that, industrial activities additionally leave behind an enormous quantity of solid waste within the variety of ash. Within the close future, additional increase within the quantity of those waste materials is anticipated to occur, particularly in Malaysia, where rising economies are expected to provide the biggest increase in energy consumption [11]. For the purpose of treating contaminated effluents, many technologies have been developed over the years. Those techniques depend on chemical precipitation, filtration, ion- exchange, reverse osmosis and membrane systems, and have their inherent advantages and limitations in application. In the last few years, adsorption has been shown to be an alternative method for removing dissolved metal ions from liquid wastes [2].
Adsorption process
Adsorption techniques for wastewater treatment have become popular in recent years due to their efficiency in the removal of pollutants that are too stable to be removed by biological methods. Adsorption is a process that occurs when a gas or liquid solute adheres to a surface (adsorbent), forming a molecular or atomic film (adsorbate). This process differs from absorption, in which a substance diffuses into a liquid or solid to form a solution. Adsorption occurs naturally, but industrialists have perfected adsorption methods to clean up hazardous waste in wastewater or purify drinking water. Adsorption has been found to be superior to other techniques for water re-use in terms of initial cost, flexibility and simplicity of design, ease of operation and insensitivity to toxic pollutants [3]. Just like surface tension, adsorption is resulted from surface energy. Atoms on the surface of adsorbent are not wholly surrounded by other adsorbent atoms. Hence, they can attract adsorbate [4]. Adsorption mechanisms are generally categorized as either physical adsorption or chemical adsorption. Physical adsorption is a result of intermolecular forces that interact between the adsorbate and the adsorbent. These physical forces include the van der Waals force consisting of weak attraction and repulsion through dipole-dipole interactions, dispersing interactions and hydrogen bonding. Dipole-dipole interactions are the result of polar compounds orienting themselves so that their charges result in a lower combined free energy. Dispersing interactions are the result of attractive forces between electrons and nuclei of molecular system. If the molecules come too close to each other, repulsive forces can push the molecules apart. Hydrogen bonding is a special case of dipole-dipole interaction in which the hydrogen atom in a molecule has a partial positive charge, attracting another atom or molecule with a partial negative charge for liquid phase systems; the van der Waals force is the primary physical force driving adsorption [5]. Physical adsorption is a readily reversible reaction and includes both mono- and multi-layer coverage. Because physical adsorption does not involve the sharing of electrons, it generally has low adsorption energy, and is not site specific. The heat of adsorption for the reaction is of an order of 40 kJ/mol mole of the adsorbate. When the intermolecular forces between a chemical molecule in a liquid stream and a solid (the adsorbent) are greater than the forces between the molecules of the liquid stream, the chemical is adsorbed onto the adsorbent surface [6]. The mechanisms of chemical adsorption are similar to those of physical adsorption, yet stronger chemical adsorption is often produced by the transfer of electrons and the formation of chemical bonds between the adsorbate and the adsorbent. It may be an irreversible reaction and have high adsorption energies. The heat of adsorption is significantly greater than for physical adsorption, ranging from 40 to 400 kJ/mol. It is not unusual for the adsorbate to have chemically changed due to the reaction. Chemical adsorption involves only monolayer coverage, and is a site specific reaction, occurring at specific functional group locations. Functional groups are distinctive arrangements of atoms in organic compounds that give the compound its specific chemical and physical properties [7]. In terms of removal of heavy metals, adsorption by activated carbon is a popular technology for treating industrial and domestic wastewater [15]. However, the high cost of activated carbon and its loss during the regeneration restricts its application. Since the 1990s, the adsorption of heavy metal ions by low-cost renewable organic materials has gained momentum [16]. The utilization of moulds, seaweeds, yeasts, other dead microbial biomass and agricultural waste materials for removal of heavy metals has been explored by Ricou-Hoeffer [17]. Consequently, there is a growing requirement for efficient, novel, and cost-effective techniques for the remediation of metal bearing wastewaters before their discharge into the environment. Over the last two decades, biosorption has been one approach showing considerable potential for metal removal from aqueous media, that is, the use of raw or natural materials and wastes from agricultural and industrial activities to adsorb metals from aqueous solutions [18]. In addition, a number of studies have evaluated the application of adsorption for the removal of Zn2+, including the use of natural materials such as bagasse [19], moss [20], bentonite [21], and mixed mineral [22]; microbial and algal biomass including seaweed, yeast, fungi, and bacteria [23]; industrial and agricultural wastes such as corncobs, peanut hulls, hazelnut shells, corn-starch, waste tea leaves, sea nodule residue, blast furnace slag, sugar beet pulp, lignite, lignin, and powdered waste sludge [3, 16, 24-29]. Despite the relative simplicity and potential cost-effectiveness of adsorption, metal removal using low-cost adsorbents is relatively unproven and needs more development before it may be applied routinely in practice.
Chemically modified adsorbens
Adsorption as one of the physicochemical treatment processes was found to be effective in removing heavy metals from aqueous solutions. It was showed that an adsorbent can be considered as cheap or low-cost if it is abundant in nature, requires little processing, and is a byproduct of waste material from waste industry. Plant wastes are cheap as they have no or very low economic value [16]. Untreated plant wastes have received a wide attention of adsorption studies such as peanut hull pellets [30], papaya stem [31], rice husk ash, and neem bark [32] saltbush (Atriplex canescens) leaves [33]. Using plant wastes for wastewater treatment has some of the advantages such as simple technique, requiring little processing, adsorption of heavy metal ions, good adsorption capacity, selective, low-cost, free availability, and easy regeneration. The chemical modification of plant wastes as adsorbents can solve several problems such as low adsorption capacity, high chemical oxygen demand (COD), biological chemical demand (BOD), and total organic carbon (TOC) due to release of soluble organic compounds contained in the plant materials. The increase of the COD, BOD, and TOC can cause depletion of oxygen content in water and can threaten the aquatic life. Therefore, plant wastes need to be chemically treated or modified before being applied to the decontamination of heavy metals. Also, pretreatment of plant wastes can extract soluble organic compounds and enhance chelating efficiency [34]. Pretreatment methods using different kinds of modifying agents have been used such as base solutions (sodium hydroxide, calcium hydroxide, and sodium carbonate), mineral and organic acid solutions (hydrochloric acid, nitric acid, sulfuric acid, tartaric acid, citric acid, and thioglycolic acid), organic compounds (ethylenediamine, formaldehyde, epichlorohydrin, and methanol), oxidizing agent (hydrogen peroxide), and dye (Reactive Orange 13). For the purpose of removing soluble organic compounds, eliminating coloration of the aqueous solutions and increasing efficiency of metal adsorption have been performed by many researchers [35-38].
Activated carbon
Activated carbon has undoubtedly been the most popular and widely used adsorbent in wastewater treatment throughout the world. Charcoal, the forerunner of modern activated carbon, has been recognized as the oldest adsorbent known in wastewater treatment. Activated carbon is produced by a process consisting of raw material dehydration and carbonization followed by activation. The product obtained is known as activated carbon and generally has a very porous structure with a large surface area ranging from 600 to 2000 m2/g [14]. Activated carbon has been found to be a versatile adsorbent, which can remove diverse types of pollutants such as metal ions [39]. In spite of better uses of activated carbon, its applications are sometimes restricted due to its higher cost. Therefore, researchers are looking for low-cost adsorbents for water pollution control, where cost factors play a major role. As such, for quite some time, efforts have been directed towards developing low-cost alternative adsorbents. Low-cost alternative adsorbents can be prepared from a wide variety of raw materials, which are abundant and cheap, having high organic (carbon) content and low inorganic content and these can be easily activated [40]. Several advantages are shown due to preparation of low-cost adsorbents from waste materials, mainly of environmental nature and economic. A wide variety of low-cost adsorbents have been prepared from different waste materials utilizing agricultural wastes as well as industrial and municipal wastes. Although many studies have been shown so far discussing the importance of low-cost adsorbents in water pollution control, many of them are generally either adsorbate specific (metals, dyes, phenols, etc.) or adsorbent specific [41, 42].
Biosorbents
Heavy metals biosorption from aqueous solutions is a relatively new process that has been verified as a very promising process in the removal of heavy metal contaminants. The major advantages of biosorption are the use of inexpensive biosorbents and its high effectiveness in reducing the heavy metal ions. Biosorption processes are particularly suitable to treat dilute heavy metal wastewater. Typical biosorbents can be derived from three sources as follows [46]: (1) nonliving biomass such as bark, lignin, shrimp, krill, squid, and crab shell; (2) algal biomass; (3) microbial biomass, for example, bacteria, fungi, and yeast. On the other hand, biosorbent process has been shown as repetitively used in cycles. The regeneration of adsorbent assents its reuse in further cycles and enables recovery of the adsorbed materials. Adsorbent regeneration can be done by specific methods which involve using chemical reagents. The metal-loaded papaya wood was completely desorbed with 0.1 mol HCl. During repeated biosorption-desorption for five cycles, no loss in the efficiency of heavy metals removal from their respective solutions and the metal-loaded biomass was noted. The study points to the potential of a novel use of papaya wood itself, a cause of environmental degradation, and otherwise of no utility for the treatment of wastewaters contaminated with heavy metals [31]. The Zn2+ ions were successively desorbed from Botrytis cinerea (B. cinerea) biomass using 10 mmol HCl solution [23]. Desorption and reusability studies indicated that the biosorbent could be regenerated with up to 98% recovery and reused five times in biosorption-desorption cycles successively.
Biomass adsorbents
Many biomass source adsorbents have been widely investigated as potential biosorbents for heavy metals. Algae, a renewable natural biomass which proliferates ubiquitously and abundantly in the littoral zones of the world, have attracted the attention of many investigators as organisms to be tested and used as new adsorbents to adsorb metal ions. Several advantages in applying algae as biosorbent include the wide availability, low-cost, high metal sorption capacity, and reasonably regular quality [45]. There are a large number of research works on the metal biosorption using algal biomass. Examples of recent reports include the biosorption of Cu2+, Cd2+, Pb2+, and Zn2+ using dried marine green macroalga (Caulerpa lentillifera) [33-36]. The biosorption of Cu2+ and Zn2+ by dried marine green macroalga (C. linum) was investigated [15, 18, 47]. At the optimum particle size (100 to 315 mm), biosorbent dosage (20 g/L), and initial solution pH 5, the dried alga produced maximum Cu2+ and Zn2+ uptake values of 1.46 and 1.97 mmol/g, respectively. Biosorbents were characteristic of broad sources, low-cost, and rapid adsorption. Unfavorably, these researches were still in the theoretic and experimental phase. Moreover, the separation of biosorbents would be difficult after adsorption.
Adsorption on natural materials
It is well understood by the study of many researchers that limestone is an effective natural geological material for the treatment of water contaminated with heavy metals. However, the reported metal removal efficiency of limestone is lower than expected or desired for intensive remediation operations, particularly with regard to the fundamental factors that affect sorption processes [6]. The influence of mechanical milling of natural Serbian clay on removal of heavy metals from an aqueous medium was investigated by Bernard et al. [7]. This study revealed that a complete adsorption of Cr and Pb, (about 98% of the initial concentration) was achieved, while Cd and Ni was adsorbed only 81% of their initial concentration. Fenglian et al. [8] found that by using natural zeolite more than 50% of Zn(II) and Mn(II) and about 60% of Fe(III) could be removed from palm oil mill effluent. Removal of heavy metals by natural materials was carried out by many researchers, some of which are listed below in Table 1.
Table 1 Adsorption capacities of modified materials for heavy metals
|
Absorbent |
Removal capacity (%) |
|||||
|
Pb2+ |
Cd2+ |
Zn2+ |
Cu2+ |
Cr6+ |
Ni2+ |
|
|
Digested raw bark (RB) |
-- |
8 |
-- |
28 |
4 |
12 |
|
Zeolite A |
99.9 |
99,9 |
99.8 |
99.9 |
-- |
99.8 |
|
Zeolite X |
99.9 |
99.9 |
99.8 |
99.9 |
-- |
99.8 |
|
Microwaved olive stone activated carbon |
-- |
96.2 |
-- |
-- |
-- |
-- |
Adsorption on industrial waste
The application of low-cost adsorbents obtained from the industrial wastes as a replacement for costly conventional methods of removing heavy metal ions from wastewater is of great interest for many researchers. Fly ash, waste slurry, red mud, lignin, sugar beet pulp, blast furnace slag, tea industry waste, and sugar cane bagasse have been proven to be a promising material. The study of two different Turkish fly ashes for their ability to remove nickel (Ni(II)), copper (Cu(II)] and zinc (Zn(II)] from an aqueous solution was done and reported that the fly ash with high calcium content (Afsin-Elbistan) was found to be a metal adsorbent as effective as activated carbon [12]. It was studied of influence of four parameters (flyy ash/lime mass ratio, type of fly ash/lime sorbent, solution temperature, and sorbent concentration) on the removal of five metallic ions (Cu2+, Ni2+, Zn2+, Cd2+, and Pb2+) [13-15]. It was found that adsorbent concentration made from fly ash, 5% by mass of lime around 122 g L-1and a solution at the temperature of 66 ℃ for a sorbent ratio of 0.66, led simultaneously to a high adsorption and a low desorption. Agro industrial solid waste jatropha husk activated carbon was use to study the feasibility of removal of toxic anions, dyes, heavy metals and organic compounds from water [14]. The study revealed that 97% of nickel, 61% of chromium IV of initial concentration could be removed. Several studies have been conducted for removal of the heavy metals from waste water by industrial waste. The removal efficiency of some of the industrial wastes is reported in Table 2.
Table 2 Adsorption capacities of industrial waste materials for heavy metals
|
Absorbent |
Removal capacity (%) |
|||||
|
Pb2+ |
Cd2+ |
Zn2+ |
Cu2+ |
Cr6+ |
Ni2+ |
|
|
Lime mud |
96 |
-- |
99 |
28 |
93 |
-- |
|
Waste rubber tire |
96 |
-- |
-- |
99.9 |
-- |
87 |
|
Saline slags generated during secondary aluminium melting processes |
5.45 |
0.82 |
1.03 |
0.63 |
-- |
-- |
|
Waste printed circuit boards |
-- |
60 |
-- |
-- |
-- |
-- |
Adsorbents regenerations and limitation
The adsorption process shows flexibility in design and operation and in many cases will produce high-quality treated effluent. Choosy adsorption utilizing biological materials, mineral oxides, activated carbon, and polymer resins have generated much excitement among researchers, scientists, and environmental engineers [8]. After the adsorbents are exhausted, they are either to be disposed of or regenerated for use. This depends upon the demand, economics involved, and the kind of pollutant that was adsorbed. Although adsorption is sometimes correctable, adsorbents can be regenerated by suitable desorption process. Furthermore, very few regeneration studies of adsorbents loaded with zinc are detailed in the literature [33-39]. Once zinc is recovered in the concentrated solution, the issue of how to dispose of this concentrated zinc product must be addressed. The metal ion attached on the adsorbent creates disposal problem as it is hazardous matter. This problem may be controlled to some scope by using elution methods. The elution of the heavy metals provides recovery of the metal ions in the concentrated form and the regenerated adsorbents. The concentrated metal solution may be convenient for recovery of the metal. The regenerated adsorbent may be recycled for reuse and ultimately the adsorbents must be incinerated [41-45]. This is a difficult task; the limitation is low-cost and properties of the suggested adsorbents discourage significant investment in regeneration. Adsorbent regeneration may account for a large portion of operating costs. Also, adsorbent regeneration may cause secondary pollution. Under such circumstances, a feasible solution is proposed to dispose the adsorbent and its contaminations into a sanitary landfill. In many cases, spent adsorbents are to be treated as hazardous waste and need to be incinerated (which in many countries causes a set of environmental and societal problems) [44].
Toxic heavy metals
The presence of toxic heavy metals such as chromium, copper and lead contaminants in aqueous streams, arising from the discharge of untreated metal containing effluents into water bodies, is one of the most important environmental problems. Environmental pollution is currently one of the most important issues facing humanity. It was increased exponentially in the past few years and reached alarming levels in terms of its effects on living creatures. Toxic heavy metals are considered one of the pollutants that have direct effect on man and animals [1]. Inputs of these trace metals into ecosystem are largely as a result of mining operations, refining ores, sludge disposal, fly ash from incinerators, processing of radioactive materials, metal plating, or manufacture of electrical equipment, paints, alloys, batteries, pesticides and preservatives [2]. The discharge of metallic ions in industrial effluent is of great concern because their presence and accumulation have a toxic effect on living species [3]. Heavy metals are toxic and detrimental water pollutant. Their presence not only affects human beings but also animals and vegetation because of their mobility in aqueous ecosystem, toxicity and non-biodegradability. Removal of heavy metals from the effluent is one of the major research carried out by researchers in the field of environment. Although different methods such as Ion exchange, precipitation, evaporation, membrane filtration and adsorption are used for heavy metals removal, adsorption process has attracted attention of many researchers because of low cost, design flexibility, and high efficiency. The present review is focused on the heavy metals removal based on the performance of various adsorbents such as natural materials, industrial byproduct, agricultural and biological waste, biopolymers and hydrogels. The objective of this study is to contribute in the search for low cost adsorbents and investigate the parameters that influence the adsorption of heavy metals on adsorbents. Due to increasing awareness about the environment and stringent environmental regulations, wastewater treatment has always been a key aspect of research. Water is a vital component for the economic prosperity of any country. In the coming years, the economic importance of water is expected to grow with the global economic growth, industrial development and urbanization. Industrial wastewater contains the variety of inorganic compounds which are characterized as toxic, carcinogenic and mutagenic which when persist in the environment have the potential to cause adverse effect on man and vegetation. The heavy metals present in the industrial waste water such as Pb, Cd, Cr, Ni, Zn, and As are the most toxic and perilous materials among the other toxic materials from the chemical and allied industries [1]. Hence there is a burning need for the removal of heavy metals from the wastewater. Various technologies that are currently used for the removal of heavy metals are evaporation, Ion exchange, precipitation, membrane filtration, and adsorption [2], among which adsorption process appears to be more favourable as it is low-cost, requires low maintenance, economical and is energy-efficient. In this article, the heavy metal removal performance of various adsorbents such as natural materials, industrial waste, agricultural and biological waste is reviewed.
Treatment methods for heavy metals removal
The various industrially used methods for the removal of heavy metals are ion exchange, chemical precipitation, floatation, electrochemical deposition, adsorption, etc., all of which are expensive and may even produce a large quantity of sludge that further needs treatment. Adsorption process is currently considered as one of the upcoming process for the treatment of wastewater containing heavy metals [4].
Effect of contact time on adsorption of heavy metals
The percentage reduction of adsorption is found to increase continually with time till the equilibrium is attained with saturation at 180 min. The percentage reduction of cadmium is 55-86, 60-85 and 56-79 for chicken egg shell, coconut leaf powder and papaya seeds respectively. This may be due to the active sites utilized is a larger surface area [2]. The percentage reduction of chromium is 50-85, 67-87 and 60-80 for chicken egg shell, coconut leaf and papaya seeds respectively. The decreased adsorption efficiency is due to less adequate availability of active sites on the adsorbents [5]. Then percentage reduction of lead is 59-82, 52-90 and 65-85 for chicken egg shell, coconut leaf and papaya seeds respectively. They are shown in the Fig, 1-3. The high percentage reduction of chromium, lead and cadmium is found to be 85, 82 and 86 for chicken egg shell powder which is rapidly increased. It has been observed that the percentage of cadmium adsorption increased with increasing agitation speed due to the proper contact between the metal ions in solution and the adsorbents’ binding sites which promotes effective transfer of cadmium ions to the adsorbents [6]. The high percentage reduction of chromium, lead and cadmium is found to be 87, 90 and 85 for coconut leaf powder and is rapidly increased. This increase maybe due to the activation of adsorption site takes place, leading to increased adsorption probably through surface exchange mechanism [7]. The high percentage reduction of chromium, lead and cadmium is found to be 80, 85 and 79 respectively in papaya seed powder. This may be due to the smaller size of adsorbent in the metal solution providing a greater availability of the metal ions to penetrate to the internal pore structure of the adsorbent. The lower metal uptake with larger adsorbent particles was due to the high diffusion resistance to mass transport [8]. The main objectives of this study are to modify adsorptive porous media to remove inorganic contaminants (heavy metals) such as Cu2+, Mn2+, Fe2+, Cr3+ and Zn2+ and the total organic compounds, and to clarify the effect of the activated ceramic on the hydraulic pressure and the flowrate for the horizontal flow of the wastewater.
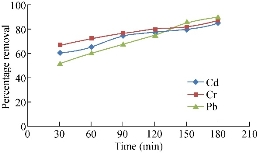
Fig. 1 Percentage removal of heavy metals by coconut leaf
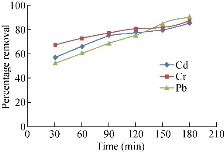
Fig. 2 Percentage removal of heavy metals by papaya seed
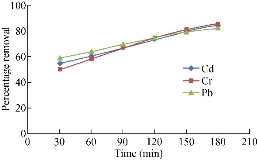
Fig. 3 Percentage removal of heavy metals by chicken egg leaf
Experimental
Laboratory model
The experimental model consisted of horizontal PVC pipes of 150 mm diameter and 6 m length, and contains manufacturing porous media (ACR). This pipe contained five points at each of 1.5 m length. At each point there is a one gauge to measure the pressure (water pressure gauge), as well as one valve for samples collection. The pipe was entirely separated and connected to the flow measurement system consisting of two parallel flow meters to control the inflow discharge. The contaminants were collected inside a 2500 L tank, and pumping to the system by electric pumps (Fig. 4 and 5). The system was built to be used for the measurements of the flow rate, pressure drop and the concentrations of Zn, Cu, Cr, Fe, and Mn in the effluents through the system.
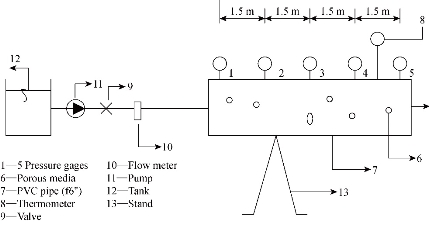
Fig. 4 Laboratory model.

Fig. 5 Laboratory model.
Manufacture porous media, activated ceramic (ACR)
Activated ceramic is a modified structural adsorptive material which had been manufactured for high adsorption process, characterized by a high porosity, a large surface area and made of mega pores interconnected by filaments, as shown in Fig. 6. This resulted in a structure with low resistance to fluid flow, making them appropriate for use as a biofilter. Powder of bentonite, kaolin and crashed coal by volume ratio 1 : 6 : 4 respectively were mixed with water by volume ratio 1 : 1 and formed thermally at different vacuum furnace temperatures. The mixture had been exposed to ozone for 30 min before activation. The effect of ozonation period and thermal treatment on physical and chemical properties of ACR under temperatures of 300, 500, 700, 900 and 1100 ℃ were studied. Grain-size distribution curves for ACR of porous media was obtained and graphed in Fig. 7. Physical properties of ACR are shown in Table 3.

Fig. 6 Activated ceramic porous media.
Table 3 Physical properties of activated ceramic Porous media.
|
Properties |
Test results |
|
Porosity |
0.37 |
|
Bulk density (kg/m3) |
1661 |
|
Particle size distribution (ASTM) Effective size d10 (mm) |
0.2 |
|
Mean grain size d50 (mm) Uniformity coefficient |
1.3 |
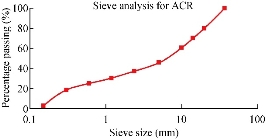
Fig. 7 Sieve size distribution curve of the activated ceramic of porous media.
Results and Discussion
Hydraulic parameters - removal efficiency relationship flow rate change
Fig. 8 shows the effect of the flowrate on the removal efficiency of heavy metals using ACR media. In this case, six gradual degrees of flowrate were used starting from 10 L/h until 100 L/h by the following steps: 10, 20, 40, 60, 80 and 100 L/h. As shown in the figures, the highest percentage removal took place with the lower flow rate 10 L/h, while the lowest was at 100 L/h. This was because when the contaminant crossed the media with a low flowrate, it would stay within the media almost enough time that helped the porous media to adsorb the contaminant in a perfect form.
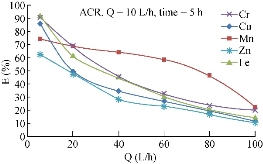
Fig. 8 Effect of flowrate on removal efficiency of the heavy metals using ACR.
Detention time
In general, the detention time depends on the porosity and the value of the contaminant’s discharge flowing through the media. Therefore, it differs from a medium to another for a same flowrate and from flowrate to another in a same media. It is expected to be increased as the porosity increases or as the flowrate decreases or as both happen. Fig. 9 shows the variation between the detention time and the removal efficiency of heavy metals in ACR media. However, it is so clear that as the detention time increased, the removal efficiency consequently increased.
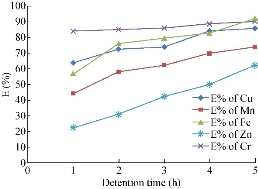
Fig. 9 The variation between the detention time and the removal efficiency of heavy metals using ACR.
Pressure drop
Fig. 9 shows the variation of pressure along the pipe through the period of the test with different flow discharges. It was shown that while the system was operating, the pressure in each point gage also continuously increased. This was because the increasing of the removable contaminant along the time would consequently result in increasing the concentrations inside the porous media. Therefore, the pressure inside the media would also increase.
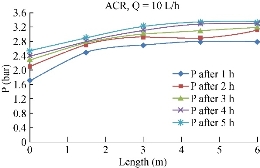
Fig. 10 The pressure distribution along the pipe using ACR.
Environmental parameters
pH values
The initial value of pH is considered the most important single parameter that influences the sorption capacity. It is related to the sorption mechanisms on the sorbent surface from water, and reflects the nature of the physicochemical interaction of the species in solution and the sorptive sites of sorbent. Fig. 6 shows the variation of pH value along the length of the pipe during the time of 5 h and a flowrate of 10 L/h. As shown in Fig. 6, in all the tests, the pH values always increased as the distance increased. This might be because the reaction had occurred between the hydrogen ions of the wastewater and the negative ions available through the porous media.
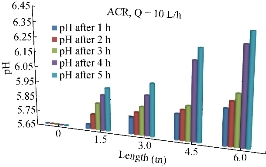
Fig. 11 The variation of pH value along the length of the pipe using ACR.
Total dissolved solid (TDS)
TDS and some other parameters including electoral conductivity (EC) were also tested for their important roles on the soil and plants. Fig. 12 shows the variations of TDS concentrations along the length of the pipe during the period of 5 h and at the discharge of 10 L/h.
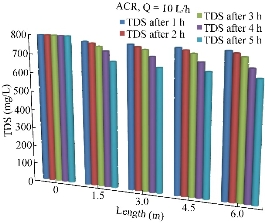
Fig. 12 Variation of the total dissolved solids (mg/l) along the pipe length using ACR.
Total suspended solid (TSS)
TSS includes all particles suspended in water which do not pass through a filter. Suspended solids are present in sanitary wastewater and many types of industrial wastewater. There are also nonpoint sources of suspended solids, such as soil erosion from agricultural and construction sites. Fig. 13 shows the variation of TSS concentrations along the length of the pipe during the period of 5 h and at the discharge of 10 L/h.
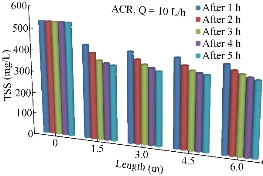
Fig. 13 Variation of the total suspended solids (mg/l) along the pipe length using ACR.
Electrical-conductivity (EC) removal efficiency
This parameter was considered for test because it is a very important parameter and has many effects on soil and plant. Fig. 14 shows the variation results of EC through the length of the pipe during the period of 5 h and at the discharge of 10 L/h.
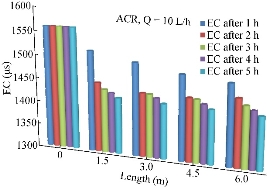
Fig. 14 Variation of EC (µs) along the pipe length using ACR.
Turbidity removal
Turbidity is another important parameter chosen to be tested too. Fig. 15 shows the variation of the turbidity with the distance of the pipe during the period of 5 h and at the discharge of 10 L/h.
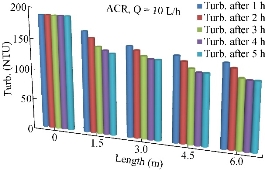
Fig. 15 Turbidity (NTU) at each length of the pipe on ACR.
Heavy metal and total organic carbon removal efficiency
Copper (Cu2+) removal efficiency
The variations of Cu2+ concentration along the length of the pipe for the period of 5 h, at the discharge of 10 L/h, with the average value of 1.65 mg/L is presented in Fig. 16 for ACR media. As shown, the removal efficiency of Cu2+ was stabled at 77% for ACR.
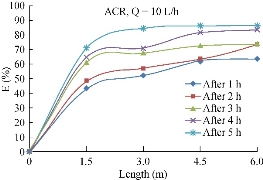
Fig. 16 Variation of copper (mg/L) removal efficiency with the length using ACR.
Zinc (Zn2+) removal efficiency
With the same condition and initial value of 0.66 mg/L for ACR media, the tests were carried and the results were represented in Fig. 17. Clarifying the figure through the time and distance, the results indicate that the maximum removal efficiency of Zn2+ reached to 52%.
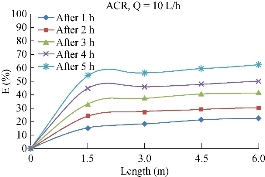
Fig. 17 Variation of Zn2+ (mg/L) removal efficiency with the length using ACR.
Chromium (Cr3+) removal efficiency
Chromium is one of the major heavy metals present in wastewater and has toxic effects on soil and plant. The results of this test are represented in Fig. 18, starting with initial values of 827 mg/L for ACR media. The maximum values of the removal efficiency of Cu2+ was noted to be about 94%.
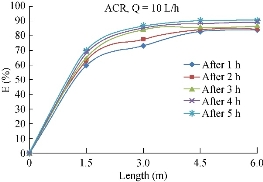
Fig. 18 Variation of Cr3+ (mg/l) removal efficiency with the length using ACR.
Manganese (Mn2+) removal efficiency
Fig. 19 shows the results with the same condition at the check point along the pipe with different operation times and starting from initial value of 20 mg/L for ACR. The figure indicates that the final removal efficiency was recorded at 95%.
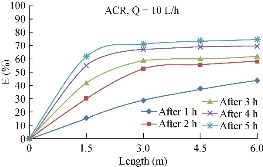
Fig. 19 Variation of Mn2+ (mg/l) removal efficiency with the length, ACR.
Iron (Fe2+) removal efficiency
Starting from initial values of 21 mg/L and the same condition above, results of this test are represented in Fig. 20. The figure indicates that the final removal efficiency was recorded at 84% for ACR.
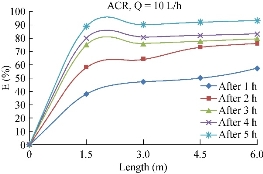
Fig. 20 Variation of Fe2+ (mg/L) removal efficiency with the length using ACR.
Total organic carbon (TOC) removal efficiency
TOC is a sum measurement of the concentration of all organic carbon atoms covalently bonded in the organic molecules of a given sample of wastewater. Fig. 6 shows the results of the test.
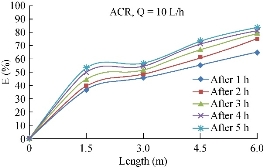
Fig. 21 Variation of TOC (mg/L) removal efficiency through time and length of the pipe.
Conclusions
This study successfully proved that the heavy metals Cu2+, Mn2+, Cr3+, Fe2+ and Zn2+ could be adsorbed from an aqueous solution in significant amounts by manufacturing porous media (ACR). It can be concluded that the removal efficiency of the heavy metals increased with the increasing of detention time, length of the pipe and pressure; the removal efficiency of the heavy metals increased with the decrease of flowrate. The results also indicate that removal efficiency using ACR was: Mn2+ ![]() Cr3+ > Fe2+ > Cu2+ > Zn2+.
Cr3+ > Fe2+ > Cu2+ > Zn2+.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Emad Abbas Jaffar Al-Mulla, Fayq Hsan Jabbar, and Rawaa Fahim Chyad AL-Hamadani. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.