Research Article
Electrochemical Characterisation of the Redox Couple of Fe(II)/Fe(III) Mediated by Nano SiO2 Modified GCE Using Cyclic Voltammetry
Muhammed Mizher Radhi 1*, Ahmed Ali Moosa 2, Ishraq Abd-Alkareem Khalaf 2
1 Radiological Techniques Department, Health and Medical Technology College-Baghdad, Middle Technology University, Baghdad, (MTU) Iraq.
2 Technical Engineering College-Baghdad, Middle Technology University, Baghdad, (MTU) Iraq.
* Corresponding author. E-mail: mmrradhi@yahoo.com
Received: Dec. 4, 2017; Accepted: Jan. 14, 2018; Published: Feb. 13, 2018
Citation: Muhammed Mizher Radhi, Ahmed Ali Moosa, and Ishraq Abd-Alkareem Khalaf, Electrochemical Characterisation of the Redox Couple of Fe(II)/Fe(III) Mediated by Nano SiO2 Modified GCE Using Cyclic Voltammetry. Nano Biomed. Eng., 2018, 10(1): 10-15.
DOI: 10.5101/nbe.v10i1.p10-15.
Abstract
A new modified working electrode of glassy carbon electrode with nanoparticles of SiO2 (SiO2 nanoparticles/GCE) was prepared by mechanical attachment method. The modified electrode (SiO2 nanoparticles/GCE) was characterised by electrochemical analysis using cyclic voltammetric technique to evaluate this electrode as nano-sensor. A standard solution of 1 mM K4[Fe(CN)6] with 1 M KCl as an electrolyte was used to study the redox current peaks of FeII/FeIII ions on the modified electrode at different concentrations, scan rates, pH, determination of diffusion coefficient (Df), reliability and stability of the modified electrode. It was found the new nano-sensor (SiO2 nanoparticles/GCE) had enhancement for the oxidation and reduction current peak of FeII/FeIII ions of about 1.29 and 1.58 µA, respectively. The current ration value of the new modified electrode was Ipa/Ipc = 1.7 with the peak separation of ∆Epa-c = 140 mV, which demonstrated that the new modified electrode acted in electrolyte as irreversible and heterogeneous reaction, had low detection limit, and enhanced the redox current peaks in acidic pH with good reliability and stability of nanoparticles on the surface of GCE.
Keywords: SiO2 nanoparticles; Cyclic voltammetry; GCE; FeII/FeIII; KCl
Introduction
Scientists studied different modified working electrodes with nanoparticles to produce high-quality sensors with low detection limit by cyclic voltammetry [1-6]. Silica (SiO2) nanoparticles were studied by electrochemical method and micro-calorimetry; the particles interacted with the monolayer of dioleoyl phosphatidylcholine on a mercury (Hg) film electrode. The result illustrated that the extent of interaction was inversely proportional to the particle dimeter. Scanning electron microscopy (SEM) images showed that the nanoparticles bound to the dioleoyl phosphatidylcholine [7]. Different photo-luminescent SiC-dots/SiO2 were synthesised by heating reaction, and the electrochemical application was studied. The electrical properties of the photoluminescence were found stable, low-toxic, low-cost and with great economic potential in many applications such as light-emitting diodes, photo-luminescent windows and fuel cells [8]. Through the electrochemical method, a silicon layer was synthesised on a molybdenum (Mo) electrode in a CaCl2 which melt containing silicon oxide (SiO2) nanoparticles at 850 oC. The oxidation of electrode-posited silicon in CaCl2 melt, producing a redox couple at positive potentials of the deposition potential [9]. SiO2 nanotubes were fabricated by hard-template growth method and evaluated as an anode for Li-ion batteries. The high-aspect-ratio character of these nanotubes allows for a relatively scalable fabrication method of nanoscale SiO2-based anodes [10]. Silicon dioxide thin films were studied with Cu/Cu+ by cyclic voltammetry. The diffusion coefficient and the mobility of Cu ions in SiO2 were calculated [11]. The synthesis of polyaniline-SiO2 composites by chemical oxidation polymerisation was carried out in the presence of phosphoric acid and was evaluated for protection of mild steel from corrosion in strong aggressive medium. A higher protection efficiency up to 99% was achieved [12]. In this study, silicon dioxide nanoparticles were used as modified materials on the glassy carbon electrode (GCE), and the electrochemical characterisation was carried out by cyclic voltammetric method.
Experimental
Materials and methods
Nano silica (20-30 nm, Hongwu International Group Ltd, China), KCl powder (SCRC, China), NaOH (BDH Company), 0.1 N HCl solution and deionise water were used in this work.
Apparatus and method
Potentiostat (EZstat series Potentiostat/Galvanostat, NuVant Systems Inc. USA) was used in the experiment. The electrochemical bio-analytical cell was connected to a potentio-stat device and monitored by the special software to perform cyclic voltammetry (CV). Silver/silver chloride reference electrode (Ag/AgCl in 3 M NaCl) was used as a reference electrode, and a platinum wire with the diameter of 1 mm as an auxiliary electrode. The GCE was used in this study after being cleaned with alumina solution and treated with ultrasonic path water for 10 min; the other working electrodes were modified by GCE with nanoparticles of SiO2 using mechanical attachment method by doping GCE with SiO2 nanoparticles [13]. The three electrodes were immersed in cyclic voltammetric cell (10 mL) with the solution. Throughout this paper, the modified GCE with nano-silica particles will be referred to as SiO2 nanoparticles (NPs)/GCE.
Results and Discussion
Enhancement of different electrodes
Different electrodes were used in electrochemical analysis by cyclic voltammetry for GCE and SiO2 NPs/GCE to be characterised by standard solution of 1mM K4[Fe(CN)6] with 1 M KCl as an electrolyte. Fig. 1 illustrates the oxidation-reduction current peaks of FeII/FeIII ions on GCE and SiO2 NPs/GCE, which shows an enhancement of both redox current peaks at modified GCE with SiO2 NPs. This nanoparticle-layer of SiO2 on the surface of GCE acted as an electro-catalyst as revealed in Table 1 [14]. Table 1 shows the enhancement of oxidation-reduction current peaks of FeII/FeIII ions in KCl solution as an electrolyte on two different electrodes. For GCE and SiO2 NPs/GCE electrodes, the oxidation-reduction enhancement of the current peak of FeII/FeIII was 1.3 and 1.6 fold, respectively. The oxidation current peak shifted to a lower potential and the reduction peak to a higher potential [15-17].
Table 1 Anodic and cathodic current peaks enhancement of modified electrodes
|
Anodic peak |
|||
|
SiO2 NPs/GCE |
Ipa = 69.2 |
Epa = 339 mV |
Enhancement = 1.3 |
|
GCE |
Ipa = 53.6 |
Epa = 339 mV |
|
|
Cathodic peak |
|||
|
SiO2 NPs/GCE |
Ipc = -41.2 |
Epc = 199 mV |
Enhancement = 1.6 |
|
GCE |
Ipc = -26.1 |
Epc = 157 mV |
|
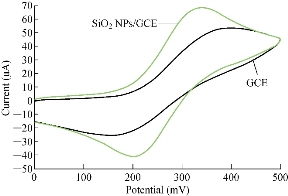
Fig. 1 Cyclic voltammogram of 1 mM K4[Fe(CN)6] in 1 M KCl on GCE and SiO2 NPs/GCE versus Ag/AgCl as reference electrode and 100 mV/sec.
Calibration graph
The characterisation of the new modified working electrode nano SiO2/GCE was carried out with different concentrations of standard solution of K4[Fe(CN)6] in 1 M KCl as the supporting electrolyte to determine the calibration curve and to calculate the detection limit of the nano sensor. Fig. 2 illustrates the cyclic voltammogram of the low and high concentrations (4-19 mM) of K4[Fe(CN)6] for the modified electrode nano SiO2/GCE to find the oxidation-reduction current peak of FeII/FeIII. Fig. 3 & 4 show the calibration graph of the oxidation-reduction peak by equations y = 0.0397x + 3.1017 with the sensitivity of R2 = 0.8508, and y = 0.0806x + 2.3135 with R2 = 0.9295, respectively. The new modified electrode SiO2 NPs/GCE had good detection limit, and so it can be used in the electrochemical analysis of samples of material traces in different concentrations. This is in agreement with the work by Orata et al. [18].
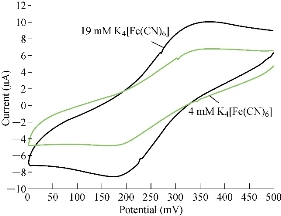
Fig. 2 Cyclic voltammogram of K4[Fe(CN)6] at different concentrations (4-19 mM) in 1 M KCl on SiO2 NPs/GCE versus Ag/AgCl as a reference electrode at 100 mV/sec.
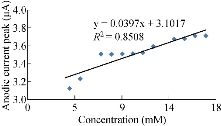
Fig. 3 Relationship between anodic current peak at different concentrations of K4[Fe(CN)6] in 1 M KCl on SiO2 NPs/GCE.
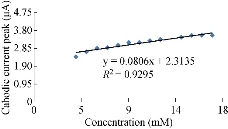
Fig. 4 Relationship between cathodic current peak at different concentrations of K4[Fe(CN)6] in 1 M KCl on SiO2 NPs/GCE.
Effect of scan rate (SR)
One important study in cyclic voltammetric technique is the different scan rates on SiO2 NPs/GCE which effected on the redox current peaks of FeII/FeIII ions by enhancing the oxidation current peak from 23.2 to 68.7 μA at scan rate 0.1 V/sec, and for reduction current peak from -14.2 to -41.2 μA at scan rate 0.01 V/sec as shown in Fig. 5. Fig. 6 shows the relationship between the oxidation current peak against the scan rate as in the equation y = 423.64 x + 21.14 with a good sensitivity of R2=95.09. Fig. 7 shows the reduction current peak of FeIII/FeII as in the equation y = 264.06 x + 15.747 with a good sensitivity of R2 = 9155. This is in agreement with the work done by of Orata et al. and Abdullah et al. [18, 19].
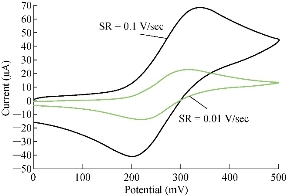
Fig. 5 Cyclic voltammogram of 1 mM K4[Fe(CN)6] in 1 M KCl on modified SiO2 NPs/GCE versus Ag/AgCl as the reference electrode at different scan rates (0.01-0.1 V/sec).
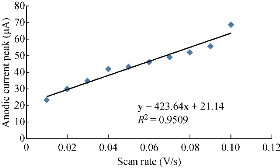
Fig. 6 Relationship between the oxidation current peak of 1 mM K4[Fe(CN)6] in 1 M KCl on SiO2 NPs/GCE versus different scan rates (0.01-0.1 V/sec).
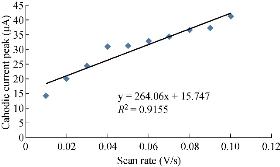
Fig. 7 Relationship between the reduction current peak of 1 mM K4[Fe(CN)6] in 1 M KCl on SiO2 NPs/GCE versus different scan rates (0.01-0.1 V/sec).
Diffusion coefficient determination
The diffusion coefficient of the redox process for FeII/FeIII ions in the 1 M KCl as an electrolyte was calculated from the Randles-Seveik equation which describes it as a reversible redox couple [20, 21]:
Ip = (2.69 × 105) n3/2 AC Df1/2 v1/2 (1)
where, Ip is the current peak (μA), n is the number of moles of electrons transferred in the reaction, A is the area of the electrode (cm2), Df is the diffusion coefficient (cm2/sec), and V is the scan rate of the applied potential (V/sec).
It was found the diffusion coefficient of oxidation-reduction reaction of FeII/FeIII ions in KCl solution on SiO2 NPs/GCE was Dfa = 2.0 × 10-5 and Dfc = 7.3 × 10-6 cm2/sec respectively, while the diffusion coefficient values of the oxidation-reduction ions on GCE were Dfa = 1.1 × 10-5 and Dfc = 1.9 × 10-6 cm2/sec. The diffusion coefficient on the modified electrode was higher than on the GCE, which indicated that the thin film of nano-materials on the surface of working electrode acted as an electro-catalyst in the electrolyte [22].
Different pH study
It is scientifically known that different pH values effect directly on the oxidation-reduction current peaks of FeII/FeIII ions in KCl as electrolyte on nano-sensors SiO2 NPs/GCE [23]. The electrochemical analysis of FeII/FeIII ions in both acidic (pH = 2) and alkaline (pH = 12) is shown in Fig. 8, which reveals the acidic medium enhanced redox current peaks about two folds than that in alkaline medium. Hence, acidic pH acted as an electro-catalyst on the surface of GCE by SiO2 NPs layer. Fig. 9 & 10 show the electrochemical behaviour of oxidation-reduction current peaks of FeII/FeIII ions in acidic and alkaline media respectively [24]. But both redox current peaks began to decline gradually after the rise of pH to 12 due to the bulky molecule and the difficulty of transmission through the electrolyte [25].
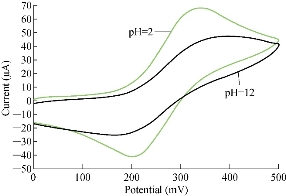
Fig. 8 Cyclic voltammogram of 1 mM K4[Fe(CN)6] in 1 M KCl on modified SiO2 NPs/GCE at different pH values (2-12 ).
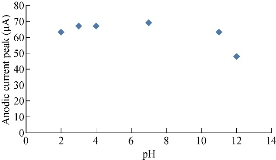
Fig. 9 Relationship between the oxidation current peak of 1 mM K4[Fe(CN)6] in 1 M KCl on SiO2 NPs/GCE versus different pH values (2-12).
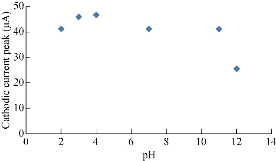
Fig. 10 Relationship between the reduction current peak of 1 mM K4[Fe(CN)6] in 1 M KCl on SiO2 NPs/GCE versus different pH values (2-12).
Reliability and stability study
Fig. 11 shows the reliability and stability of nanoparticles of SiO2 on the surface of GCE in electrolyte using cyclic voltammetric technique. The relative standard deviation (RSD) was determined from the range of oxidiation-reduction current peak values at 10 times by cycling voltammogram with accepted results of ± 0.5% and ± 0.61%, respectively which was in agreement with the work of Karimi et al. and Naseri et al. [25, 26].
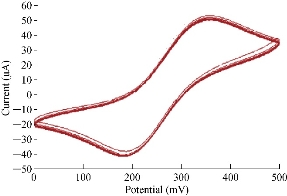
Fig. 11 Cyclic voltammogram of 1 mM K4[Fe(CN)6] in 1 M KCl on SiO2 NPs/GCE at 10 times.
Transmission electron microscopy (TEM) study
Fig. 12 shows the morphology structure of SiO2 NPs by TEM. It was found that the spherical shape of nanoparticles was in fine picture with dimeters of 20-30 nm.
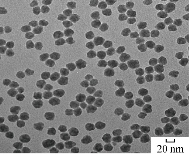
Fig. 12 TEM of SiO2 NPs.
Conclusions
It can be said that the modified working electrode as prepared in the laboratory, the nano-sensor SiO2 NPs/GCE, had good electrochemical properties in the cyclic voltammetric analysis. The results also indicated that the new modified electrode had good reliability, stability, high enhancement in conductivity and low detection limit, acted as electro-catalyst in acidic pH, and showed irreversible heterogeneous process in aqueous solution. Diffusion coefficient values of redox current peaks of FeII/FeIII ions on the modified electrode were calculated from Randel equation, which was Dfa = 2.0 × 10-5 cm2/sec and Dfc = 7.3 × 10-6 cm2/sec. It appeared that the silica micro-particles were non-conductive, but conductive in their nano dimeter, and the conductivity of the working electrode was enhanced in cyclic voltammetry.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Muhammed Mizher Radhi, Ahmed Ali Moosa, and Ishraq Abd-Alkareem Khalaf. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.