Research Article
Enhanced Peptide Delivery and Sustainable Release of Pleurocidin Using N-Succinyl Chitosan Nanoparticle
Giritharan Bupesh 1, 2*, Chinnaiyah Amutha 2, 6, Sakthivel Vasanth 1, 2, Tharaumasivam Siva Vijayakumar 2, 5, Kannaiyah Pandian 3*, Vellingiri Balachandar 4*, Durai Rajan Gunasekaran 1
1 R&D Wing, Central Research Laboratory, Sree Balaji Medical College & Hospital (SBMCH), BIHER-Bharath University, Chrompet, Chennai-600 044, India.
2 Department of Animal Science, Bharathidasan University, Tiruchirappalli, Tamil Nadu, India.
3 Department of Inorganic Chemistry, University of Madras, Guindy Campus, Tamil Nadu, India.
4 Department of Human Genetics & Molecular Biology, Bharathiar University, Coimbatore-641046, Tamil Nadu, India.
5 Department of Biotechnology, Srimath Andavar College, Srirangam, Tamil Nadu, India.
6 Department of Animal behavior & Physiology, Madurai Kamaraj University, Madurai, Tamil Nadu, India.
* Corresponding authors. E-mail: bupeshgiri55@gmail.com; jeevapandian@yahoo.co.uk; geneticbala@gmail.com
Received: Nov. 29, 2017; Accepted: Dec. 12, 2017; Published: Dec. 29, 2017
Citation: Giritharan Bupesh, Chinnaiyah Amutha, Sakthivel Vasanth, Tharaumasivam Siva Vijayakumar, Kannaiyah Pandian, Vellingiri Balachandar, and Durai Rajan Gunasekaran, Enhanced Peptide Delivery and Sustainable Release of Pleurocidin Using N-Succinyl Chitosan Nanoparticle. Nano Biomed. Eng., 2017, 9(4): 324-332.
DOI: 10.5101/nbe.v9i4.p324-332.
Abstract
Chitosan is a natural polymer that can be modified into chemically different types. In order to imply biomedical applications, the chitosan dissolved in aqueous medium. Therefore, in the present study, functionally substituted chemical groups such as O-carboxymethyl chitosan (CMC), N-succinyl chitosan (NSC), quaternary ammonium chitosan (QAC) and tripoly phosphate chitosan (TPC) were synthesized. The peptide derived from Clarias batrachus was used as a drug that is successfully entrapped with NSC nanoparticles and were characterized by FTIR, AFM, SEM, Zeta sizer, fluorescent imaging and SDS-PAGE. In addition, antimicrobial activity was examined. The results of NSC obtained particle size (~64 nm) with highest solubility and 80% of antimicrobial activity against multidrug resistant pathogen Klebsiella pneumonia. Finally, the release profile indicates that the NSC nanoparticles deliver the peptide with 75% sustainable release and good availability up to 96 h. The current research was the first to report the characterization of peptide with NSC nanoparticles by SDS-PAGE, in which the antimicrobial activity was enhanced up to 80%, the particle sizes were obtained ~64 nm from SEM and AFM, and NSC-peptide exhibited 75% of sustainable release and good availability up to 96 h.
Keywords: Chitosan; Peptide delivery; Encapsulation; N-succinyl chitosan; Clarias batrachus; Antimicrobial activity
Introduction
Peptide drug delivery is still very hard task to deliver to the target tissue by specific and nonspecific release mechanisms. It could be due to poor absorption and susceptibility to enzymatic degradation. Polymers were promising carriers for application of drug delivery. Polymers have good compatibility to deliver drugs, over the routine method of microspheres [1-3]. It has been perceived to navigate the epithelium is greater than the number of microspheres breaching the epithelium. Biodegradable nanoparticulate systems have significantly drawn considerable attention for potential drug delivery vehicle. Chitosan is excellent biopolymer that is biocompatible and biodegradable [4-6]. Generally, the pharmaceutical and medical fields use chitosan for its good biocompatibility such as haemostatic, bacteriostatic, fungistatic, anticarcinogenic, low toxic and anticholesteremic properties [7]. The protonated amine groups in chitosan allowed the transport of drugs across cellular membrane [8, 9] and subsequent endocytosis into cells [10]. Chitosan and its derivatives are excellent and effective biopolymers bear special features like mucoadhesive and absorption. Therefore, it is extensively studied for delivery of therapeutic proteins and antigens specifically via mucosal routes. Chitosan and its subordinates ready to collaborate with bodily fluid and epithelial cells and to initiate the cytoplasmic F-actin and junction protein resulting to open cell tight junctions and expanding the permeability of para cellular epithelium [11-13]. Besides, the charge and other structural elements of the polymers have facilitated the membrane permeation activity [14]. In many studies, the chitosan-based formulations are superior to enhance the absorption of therapeutic peptides and antibodies in post mucosal vaccination [15-19]. Nano-encapsulation is excellent technique for the drug formulation that has many advantages, such as controlled release, site-specific drug delivery, minimizing side effects, and protecting sensitive drugs [20, 21]. Further, the CS-NPs which obtained by a very mild ionotropic gelation procedure have a long shelf life have been reported to possess excellent capacity for the association of proteins [22-24]. However, chitosan nanoparticles were seldom reported for antibacterial activity. Therefore, the present study is intended to investigate the synthesis of various types of chitosan, screening their antimicrobial efficacy, characterization of N-succinyl chitosan (NSC) and its encapsulation of Pleurocidin peptide. This novel finding could expect to form a pilot model for peptide drug delivery.
Experimental
Preparation of various chitosan
Chitosan sulphate purchased from Sigma-Aldrich, Bangalore, India was used in the synthesis of various chitosan by chemical route. Carboxymethyl chitosan (CMC), NSC, quaternary ammonium chitosan (QAC) and tripolyphosphate chitosan (TPC) were synthesized by employing the following different methods. The NSC nanoparticles were synthesized according to Zhu et al. [25]; CMC was synthesized according to Wu et al. [26]; QAC and TPC were synthesized by ionic gelation method following Calvo et al. and Zhang et al. [27, 28].
Peptide
The non-specific defense peptide was used in this study for peptide delivery. The peptide was isolated from Clarias batrachus, which was characterized by MALDI-TOF and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The purified peptide was separated by high performance liquid chromatography pooled and lyophilized into powder used as peptide source.
Evaluation of antibacterial activity
The chosen bacterial cultures were incubated at 37 °C in bacterial incubator. At the exponential phase, bacteria were harvested by centrifuge at 4000 g for 10 min at 4 °C, and then washed twice with 10 mM phosphate buffer saline (PBS, pH 7.2). The bacteria were suspended in PBS and adjusted to 1 × 107 CFU/mL for further use. The minimum inhibitory concentration (MIC) was determined by Avadi et al. [29, 37]. All chitosan was deciphered in 0.25% acetic acid. The used microorganism was K. pneumonia. The experiments were performed in triplicates and ciproflacin used as a standard drug for K. pneumonia. Three concentrations (10, 25 and 50 mg concentration) of NSC and the standard ciproflacin were prepared in water. A standard concentration of K. pneumonia 1 × 107 CFU/mL was uniformly inoculated on the surface of an agar plate. Consequently, five discs each impregnated with synthesized chitosan, placed on the surface of the medium. During incubation, polymers expelled out and penetrated into the media depending on their size, concentration gradient around each disc. Drug loaded disc showed zone of inhibiton, reflecting the degree of susceptibility to the microorganism of respected polymer [37, 38].
Characterization of NSC nanoparticles
The morphology was observed using atomic force microscope (AFM). An aqueous solution was placed on a clean mica substratum and purged with nitrogen. The microscopic images were obtained by a Nano-R2™ AFM (Pacific Nanotechnology, USA) in a scanned area of 1.2×1.2 µm. Scanning electron microscopy (SEM) was employed with Electron microscope (LEO Electron Microscopy Inc., Thornwood, NY) operating between 1 and 3 kV with a filament supplied with 0.5 mA volt. Liquid samples were deposited on vitreous carbon stubs and dried at room temperature. The samples were coated with a palladium–platinum layer with 4 nm using a Cressington sputter-coater 208HR with a rotary-planetary-tilt stage, equipped with MTM-20 thickness controller. The size (Z-average mean of triplicate) and zeta potential of the were analyzed by photon correlation spectroscopy and laser doppler anemometry, using a Zetasizer 3000HS (Malvern Instruments, UK). Fourier transform infrared spectroscopy (FTIR) fingerprint spectra were recorded using Shimadzu IR-Spectrophotometer (Model 8000) within the wave number range of 4000–600/cm at 25 °C. The NSC and the peptide encapsulated nano particles were stained with 0.5%. Imaging was performed in glass slide using an XSP-11CE microscope with a 100 × oil immersion objective lens in dark field mode.
Determination of peptide encapsulation efficiency and loading capacity
The encapsulating efficiency and loading capacity of peptide with the different formation were determined by ultra centrifugation of samples at 16,000×g and 15ºC for 30 min and the amount of free peptide was determined in clear supernatant by UV-Spectrometry at 280 nm using supernatant of unloaded as basic correction. The peptide Loading Capacity (LC) of and Association Efficiency (AE) of the process were calculated as follows:
![]() (1)
(1)
![]() (2)
(2)
Peptide loading profiles by in vitro assay
The in vitro peptide loading profiles of chitosan were determined as follows. The peptide pleurocidin loaded chitosan was separated from 12 mL suspension. It was then placed into test tubes with 4 mL of 0.2 mol/L PBS of pH 7.4 and incubated at 37 ºC under constant stirring. At the periodic intervals of (12, 24, 36, 48, 72 and 96 h), the samples were centrifuged and fresh medium (2 mL) were deciphered after the subsequent removal of supernatant. The quantity of peptide released evaluated by modified Coomassie brilliant blue protein assay. The calibration curve was developed using non-loaded peptide as correction.
In vitro peptide release studies
About 10 mg of the dried peptide loaded was suspended in 2 mL of 0.1 mol/L HCl or 0.1 mol/PBS of ~pH 7.4 and stabilized with 0.2% NaN3 (W/V) then incubated at 37ºC under stirring at the rate of 500 rpm on the Remi Constant temperature with shaker The samples were centrifuged at periodic intervals. 2 mL of the supernatant were taken out and free peptide (FP) was determined by protein assay. The calibration curve was made using non-loaded peptide as correction. The experiments were done in triplicate and the mean values were expressed as result. The error bars in the peptide showed the standard deviation of data.
Statistical analysis
Data were analyzed using SPSS 17 version statistics software. Table.1 shows the one-way of variance for statistical significance of the model with Dunnet comparisons to test for statistically significant effects of Free and Encapsulated chitosan’s (P < 0.05).
Results and Discussion
The various types of chitosan were synthesized viz. NSC, quaternary ammonium chitosan, tripolyphosphate chitosan, and carboxymethyl chitosan. The chitosan was first evaluated for its worthiness based on the highest antibacterial activity to enhance the therapeutic purposes by encapsulated with peptide for peptide delivery. The pleurocidin was used as a distinctive peptide for the delivery as peptide drug. The chitosan was evaluated for the antibacterial activity, and the potency was reckoned by the antibacterial screening against K. pneunonia the multidrug resistant strain shown in (Fig. 1(a)). The positive control was used as ciproflacin. Among the four chitosan, the NSC demonstrated the most noteworthy action when contrasted with alternate sorts. The chitosan demonstrated antibacterial movement bearing the dose dependent manner. Further, all the forms of chitosan were encapsulated with the peptide. Yet again, the peptide encapsulated was screened for the enhancement of the antibacterial activity (Fig. 1(b)). The peptide embodied utilizing NSC demonstrated the most astounding action when contrasted with the standard medication ciproflacin. Among the other chitosan, the quaternary ammonium chitosan showed great action. The activity was increased significantly to the dose dependent way as displayed in the (Fig. 1(a), (b) and Table 1). The encapsulated peptide and the free NSC were examined under fluorescent microscope at oil immersion field. Fig. 2 shows the sound encapsulation of peptide with the NSC. Fig. 2(a) depicts the free NSC and Fig. 2(b) peptide encapsulated with the NSC. In Fig. 2(b), some of the empty capsules were also seen within encapsulated chitosan peptide. By counting, the total number of particles, at least 50 % of chitosan was found to load with the pleurocidin-like peptide (Plp) (Fig. 2(b)). The Plp was encapsulated for the peptide delivery with NSC. This peptide was isolated from a skin of Clarias batrachus catfish which had molecular weight of 25 KD and it exhibited excellent antimicrobial property. The peptide encapsulation was done using NSC by immobilization technique through micro syringe. The Plp encapsulated was confirmed by SDS-PAGE (Fig. 3(a)). The wide protein marker was loaded in lane 4. Lane 2 demonstrates the encapsulation of peptide with NSC which was confirmed by the stuffed of spherical shaped peptide surrounded by the NSC. NSC was displayed in lane 3. The encapsulation was assessed further by ensuring the thick band in the range from 45 to 65 KDa molecular weight documented in lane 2. Lane 3 illustrates the 41 KDa molecular weight of chitosan nanoparticle alone.
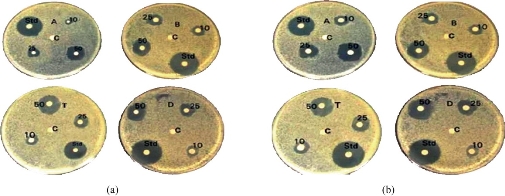
Fig. 1 (a) Antimicrobial efficiency of differently derived chitosan. A: Carboxymethyl chitosan, B: Trimethyl chitosan, C: Negative control, Std: Positive control, T: Quaternary chitosan, and D: N-succinyl chitosan. (b) Peptide encapsulated with A, B, T and D as above.
Table 1 The antibacterial effect of various chitosan nanoparticles CMC, TMC, QAC and NSC in free form and as peptide encapsulated with chitosan nanoparticle-forms against gram-positive Klebsiella pneumoniae
|
Free chitosan and encapsulated chitosan |
Zone of inhibition (mm) |
Ciproflacin (100 µg) |
||
|
10 µg |
25 µg |
50 µg |
||
|
Carboxymethyl chitosan (CMC) |
6.96 ± 0.12
|
10.06 ± 0.21 |
13.01± 0.53 |
38 mm |
|
Trimethyl chitosan (TMC) |
8.03 ± 0.18
|
11.06 ± 0.46 |
17.00 ± 0.20 |
|
|
Quatnery ammonium chitosan (QAC) |
9.98 ± 0.23
|
14.03 ± 0.18
|
19.09 ± 0.35
|
|
|
N-succinyl chitosan (NSC) |
12.03 ± 0.39
|
21.78 ± 0.26 |
29.20 ± 0.36 |
|
|
CMC + Pl-peptide |
13.10 ± 0.17
|
22.09 ± 0.08 |
31.96 ± 0.12 |
|
|
TMC + Pl-peptide |
15.01 ± 0.14
|
28.98 ± 0.39
|
32.70 ± 0.45
|
|
|
QAC + Pl-peptide |
17.03 ± 0.12
|
31.03 ± 0.12
|
37.00 ± 0.11
|
|
|
NSC + Pl-peptide |
19.01 ± 0.18
|
33.99 ± 0.11
|
43.00 ± 0.28**
|
|
Note: **Values were significant in one-way ANOVA, Duncan’s test; p < 0.05.
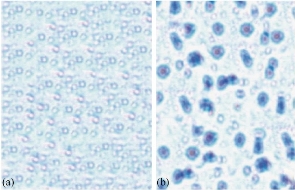
Fig. 2 Encapsulated pleurocidin-like peptide with NSC and free NSC nanoparticle under fluorescent Microscope: (a) free NSC; and (b) pleurocidin-like peptide encapsulated with NSC.
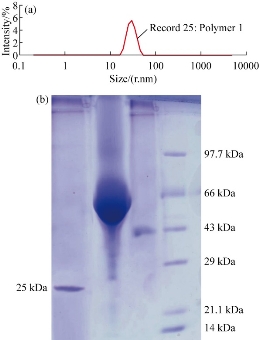
Fig. 3 (a) SDS-PAGE showing standard molecular weight marker: L1 = Pl peptide; L2 = encapsulated Pl-peptide within NSC; L3 = NSC nanoparticles; and M = protein marker. (b) Average size distribution of chitosan in particles size analyzer.
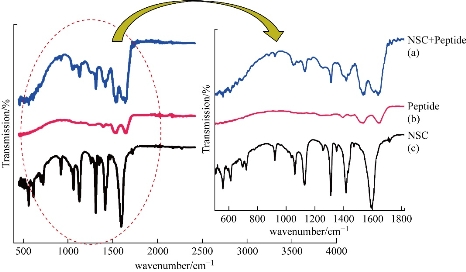
Fig. 4 FTIR spectra of (a) NSC + peptide, (b) peptide and (c) NSC alone.
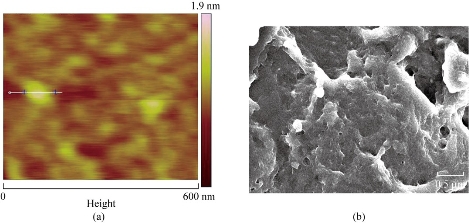
Fig. 5 (a) AFM image of NSC nanoparticles showing size of 64 nm. (b) SEM image of NSC nanoparticles depicting long-chain carrier with compartmental architecture.
Particle size distribution
The mean size and size distribution of synthesized chitosan were characterized by using Zetasizer. The size distribution profiles are as shown in (Fig. 3(b)) and these results represent a typical batch with a mean diameter (64 nm) and a narrow size distribution (polydispersity index < 1). The chitosan particles size distribution was further confirmed from AFM studies. From the analysis, the individual particles can be calculated.
FTIR
The FTIR spectra of synthesized NSC are as shown in Fig. 4: Spectra of (a) NSC nanoparticle, (b) peptide and (c) peptide encapsulated with NSC. Fig. 4(a) shows the chitosan spectrum, and it was found that these distinctive absorption bands appear at 1662/cm (Amide I), 1599/cm (–NH2 bending) and 1380/cm (Amide III). The absorption bands at 1156/cm (asymmetric stretching of the C–O–C bond), 1075 and 1033/cm (skeletal vibration involving the C–O stretching) are the characteristics of saccharine structure. In the Fig. 4(b), compared with that of chitosan, CH2 was found in 2417/cm. The peak 2900 represents the presence of C=O. The 1599/cm interpret the N-H bonds which are involved in the hydrogen bonding that shifts the different elements of secondary structure that are observed (the lower wavenumbers region are expanded and inset). Fig. 4(c) demonstrates that the amino stretch declined greatly, and the peak at 1656/cm showed amide I and 1380/cm shows amide III that confirmed the content of the secondary structure of protein. The spectrum at the peak indicates the succinyl derivation reaction took place at the N-position. The spectrum shows the –NH–CO–groups which bounded to the peptide and the stretch was formed. No other new impurity peaks were not observed in the peptide encapsulated with NSC. This settles the encapsulation of Pl-peptide with the NSC.
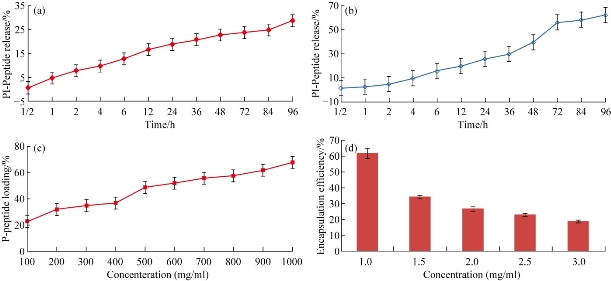
Fig. 6 In vitro pleurocidin-like peptide release from NSC in different media: (a) in 0.1 mol HCl and (b) in 0.1 mol PBS (pH 7.4) (NSC Mw=45 Kda, N=6). (c) Pleurocidin-like peptide loading efficiency with NSC nanoparticles. (d) Pleurocidin-like peptide encapsulation effieciency on different concentrations of NSC. (Each column represents the mean ± SEM n=6.)
SEM analysis
The NSC was further characterized by SEM as shown in (Fig. 5(b)). The macroscopic chitosan particles appeared as a long chain of interacting particles (Fig. 5(a)) but at a higher magnification in AFM. These deacetylated polymers appeared as composed of nanoparticle calculated diameter of 60 (±6) nm. Surface topography of NSC is as shown in AFM image (Fig. 5(b)) and demonstrated the exact particle size distributed in the chitosan nanoparticles (~ 64 nm). Peptide loading capacity is an important factor for releasing property of nano-delivery. The release rate is depending on the driven drug concentration. Higher levels of loaded drug leads to a wider concentration gap between the polymeric nanospheres and the release medium which cause a higher diffusion rate. The in vitro peptide release studies are as shown in (Fig. 6(a)-(d)). It was influenced by the quantity of protein entrapped or encapsulated and the maximum loading capacity provided a fast release rate. Difference in the release rate was attributed in the PBS as 62 % of loading capacity of peptide concentration gradient (Fig. 6(b)). The higher NSC focus gives the most noteworthy discharge rate (Fig. 6(c)) which indicates coordinate relationship. The embodiment proficiency and the arrival of various chitosan fixation concur in the discharge consider. Both the concentration of chitosan and peptide act as a vital factor for loading capacity. In Fig. 6(c) and (d) the drug release rate is the drug concentration gradient driven and the higher levels of drug loaded leads to initial higher diffusion rate which causes the observed initial difference. However, later 48 h the amount of drug entrapped gradually decreases through the membrane permeability and becomes main factor for controlling the release profile. Higher concentration of drug resulted in higher permeability to measure and to promote Plp release. However, Fig. 6(d) reveals the lowest carrier concentration (1 mg/mL) achieved the highest encapsulation efficiency. Therapeutic proteins were treated for many diseases and disorders, which are increasingly becoming a very important class of medication, in this era and rapid growth in the area of pharmaceuticals industry. The growing interest and recent modern tools in physiology and therapy as well as the established capability of producing a large quality by sophisticated nanobiotechnological strategies. The peptide drugs were easily degraded through the proteolytic enzymes from gastrointestinal tract that was short acting for repeated injections that are often required for the viable delivery system to improve their systemic bioavailability, biocompatibility and to increase the half-life periods. Therefore, developing an effective and novel delivery system for the peptides and various compounds has raised an increased attention. A successful system should not only protect the drugs from enzymatic degradation but also increase the permeability to target the gastrointestinal barriers. In spite of tremendous examinations, the oral bioavailability of peptide drug like insulin demonstrated very low and regularly inadequate to have the fundamental impact [30,31]. Thus, the present work gives a novel solution for the effective therapeutic peptide delivery strategy via encapsulation of peptide into nano-particulate carriers. This framework would secure the peptide drugs against chemical and enzymatic corruption and furthermore conceivably to upgrade the specific take-up of these particles by different cells [32]. By compared with all synthesized chitosan, the NSC showed effective killing of bacterial and fungal pathogens. The synthesis strategy was prepared at room temperature especially in the final step of down streaming the particles were kept at 40 ºC without using sonication. Thus, it is an excellent procedure to encapsulate the protein and peptide drugs that are sensitive to different stress factors [33]. Synthesis of several novel characteristics like good cellular uptake, colloidal stability, definite surface charge, morphology and distribution. The SDS-PAGE of the peptide embodied with the NSC nanoparticles demonstrated that the knock of peptide encompassed by the NPs. The atomic force microscopic (AFM) image showed the spherical like-morphology was observed around 60 nm, which is also confirmed by SEM studies. The zeta particle sizer measurements revealed that the colloidal stability of the NSC. Another major advantage of this technique indicated is more than 80 % encapsulation efficiency was achieved in the NSC nanoparticles. The in vitro and in vivo conduct of nanoparticle grades to be extraordinarily conquered by their physicochemical properties [34]. The NSC infers particles size (64 nm) and shows in the shape of spherical with a polymer network of mesh like structure observed in the SEM study. The encapsulation was principally made by the property of the carriers and drug particles. In the present examination, the polymer shows the positive charge and the peptide drug has negative charge. This suggests the peptide was embodied towards the nanocarrier NSC. The principle involved in this encapsulation was ionic interaction and the surface charge of the peptide. The chitosan nanoparticle exhibited the opposite charge that made a strong affinity in the protein drug encapsulation. This typical type of work has been made an attempt in doxorubicin [35, 36]; however, in the peptide drugs work has been made a first attempt for encapsulating the Plp antibiotics. Finally, the present work exhibits the peptide drug delivery through nano measured molecule transporter; NSC demonstrates a maintained discharge up to 96 h showing that a decent bioavailability and a fundamental discharge communicated a knowledgeable fuse of peptide drug encapsulation complex. Moreover, there was a significant difference in release profile at 0.1M PBS at pH 7.4 and at 0.1M HCl on peptide release from NSC nanoparticle. The peptide release was highly increased in PBS when compared to HCl. Therefore, the present model can be used efficiently for development of peptide drugs for therapeutics use for multidrug resistant K. pneumonia infection.
Conclusions
It is discernable that synthesized (64 nm) N-succinylchitosan np’s acts as a good carrier for peptide delivery. Among the bio property of chemically varied chitosan, the NSC revealed the highest antimicrobial activity. The peptide encapsulated NSC also enhances the strong antimicrobial sensitivity against K. pneumonia. The NSC (64 nm) encapsulated with peptide were characterized by AFM, FTIR, SEM and SDS-PAGE. The SDS-PAGE technique was first attempted for the confirmation of peptide-NSC encapsulation. The release profiles of NSC with Plp evidenced the availability and sustainable release up to 96 h. The peptide release was significantly increased at 0.1 mol PBS media at pH 7.4 than at 0.1 mol HCl media. Therefore, the present model can be used as an ideal study for the development of peptide drug delivery for therapeutics use for multidrug resistant organisms (K. pneumonia) infection in the near future.
Acknowledgments
The authors would like to express their deep gratitude to UGC-SAP, DST-FIST, ICMR, Moe&F and DOD, Govt. of India, Department of Animal Science, Bharathidasan University, Tiruchirapalli, Tamilnadu, and Sree Balaji Medical College and Hospital, Chromepet, India for providing the adequate laboratory facilities in the successful completion of this research work.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Giritharan Bupesh, Chinnaiyah Amutha, Sakthivel Vasanth, Tharaumasivam Siva Vijayakumar, Kannaiyah Pandian, Vellingiri Balachandar, and Durai Rajan Gunasekaran. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.