Research Article
A Comparative Study of Plant Mediated Synthesis of Silver Nanoparticles from Fresh Leaf Extracts of Gracina Gummi-Gutta L., Cynodon Dactylon L. and Bauhinia Acuminata and Their Antimicrobial Activity Studies
Asha R. Pai 1, 2*, Anantha M. Pillai 2, Abhijith Jayapraksh 2, Ajith John 2
1 Dept. of Physics, Amrita School of Arts and Sciences, Amrita Vishwa Vidyapeetham, Amritapuri, Kollam -690525.
2 Amrita School of Biotechnology, Amrita Vishwa Vidyapeetham, Amritapuri, Kollam – 690525.
* Corresponding author. E-mail: asharp@am.amrita.edu
Received: Oct. 27, 2016; Accepted: Dec. 9, 2016; Published: Dec. 21, 2016.
Citation: Asha R. Pai, Anantha M. Pillai, Abhijith Jayapraksh, and Ajith John, A Comparative Study of Plant Mediated Synthesis of Silver Nanoparticles from Fresh Leaf Extracts of Gracina Gummi-Gutta L., Cynodon Dactylon L. and Bauhinia Acuminata and Their Antimicrobial Activity Studies. Nano Biomed. Eng., 2016, 8(4): 288-296.
DOI: 10.5101/nbe.v8i4.p288-296.
Abstract
Metallic nanoparticles traditionally synthesized by wet chemical synthesis techniques used chemicals used which were quite often toxic and flammable. So a reliable and eco-friendly process for synthesis of metallic nanoparticles has become an important step in the field of nanotechnology. Plant extracts are eco-friendly and are economic and efficient alternatives for the large-scale synthesis of nanoparticles. The present study is planned to compare the biosynthesis of silver nanoparticles using the fresh leaf extracts of three potential plants with high medicinal value such as Gracinia gummi gutta L. (Gracinia cambogia), Cynodon dactylon L. and Bauhinia acuminata. A rapid reduction of silver ions leading to the formation of stable silver nanoparticles in solution in 5 minutes at room temperature as compared to that on heating was studied. The UV-Visible spectrum of silver nanoparticles in aqueous solution shows an absorbance peak around 435 nm due to surface plasmon resonance. Transmission electron microscope images showed the particle size as around 10 nm and their hydrodynamic size were obtained to be around 100 nm. This is a first attempt to perform eco-friendly synthesis of silver nanoparticles using fresh green leaves of Gracinia gummi-gutta L and produced the maximum yield which may benefit various industries with wide range of applications. These biologically synthesized silver nanoparticles were tested for antibacterial activity against three human pathogens such as Escherichia coli, Pseudomonas aeroginosa, and Staphylococcus aureus. An effective synthesis of silver nanoparticles using aqueous leaf extracts of Gracinia gummi-gutta L. was there by investigated.
Keywords: Silver nanoparticles; Gracinia gummi-gutta L.; Cynodon dactylon; Bauhinia acuminata; TEM; Hydrodynamic size
Introduction
Metallic nanomaterials have the potential ability to alter and improve the pharmacokinetic and pharmaco dynamic properties of various types of drug molecules. The metallic stable dispersions of especially gold, silver and copper are highly useful in areas such as microbiology, surface enhanced Raman scattering (SERS) detection, photography, catalysis, biological labeling, photonics and optoelectronics. Silver nanoparticles are of particular interest based on their applications in medical devices and healthcare products because of their antibacterial activity and low toxicity to the human cells. In medicine, silver and silver nanoparticles have ample applications such as skin ointments and creams which contain silver to prevent infection of burns and open wounds [1], medical devices and implants prepared with silver-impregnated polymers [2]. Therefore there is still a need of economic commercially viable as well as environmentally clean synthesis routes to synthesis these nanoparticles. On the other hand it is seen that the plant extracts, being safer to handle and a source of several metabolites [3], are eco-friendly, economical and cost effective for synthesis of large scale of nanoparticles compared to the other biological synthesis methods using fungi or microbial cultures [4,5]. Many reports are available on the biogenesis of silver nanoparticles using several plant extracts, such as Zea mays [6], Azadirachta indica (Neem) [7], Medicago sativa (Alfa alfa) [8,9], Aloe vera [10], Emblica officinalis (Amla) [3], Capsicum annuum [11], Geranium sp. [12, 13], Diopyros kaki [14], Magnolia kobus [15], Coriandrum sp. [16], Catharanthusroseus [17], Stigmaphyllonlittorale [18], etc. In this investigation we present a comparative study of the rapid green synthesis of silver nanoparticles (Ag NPs) from green extracts of Bauhinia acuminata, Cynodon dactylon and Gracinia gummi-gutta L. and their characterisation and their inhibitory effects against Gram negative and Gram positive bacteria. Bauhinia acuminata (Fabaceae family, sub family – Caesalpinioideae) is a semi-deciduous large shrub with white butterfly like flowers [19]. This species occurs widely in deciduous forests and scrub. Other common names include Dwarf white bauhinia (English), Safed Kachnar (Hindi) and Sivamalli (Sanskrit). Cynodon dactylon L. (Poaceae family, sub family – Chloridoideae) is a perennial weedy grass and is one of the ten auspicious herbs that constitute the group ‘Dasapushpam’ in Ayurveda [20]. It is reported to be the most sacred plant of India next to Ocimum [21]. Common names around the world include Bermuda grass (English), Doorwa (Sanskrit). Garcinia cambogia or Garcinia gummi-gutta L (Clusiaceae family) [22] is a tropical species native to Indonesia. This plant is grown for its fruit which looks like a small pumkin in Southeast Asia, coastal Karnataka/ Kerala, India, and west and central Africa. Along the west coast of South India it is popularly termed as Malabar tamarind. The extract and rind of G. gummi-gutta is a curry condiment in India especially for its sour flavors and is also purported as a potential antiobesity agent.
Materials and Methods
Silver nitrate was purchased from Merck India Ltd (99%). The fresh green leaves of Bauhinia acuminata, Cynodon dactylon L., Garcinia gummi-gutta L. were obtained from the neighbourhood of Amrita School of Biotechnology, Amritapuri, Kerala, India. Pseudomonas aureginosa (Rod shaped gram negative bacteria), E coli (gram negative bacteria) and Staphylococcus aureus (gram positive bacteria) were cultured and used for inhibitory studies.
Fig. 1 Green leaves of (a) Bauhinia acuminata, (b) Cynodon dactylon L., and (c) Garcinia gummi-gutta L.
Preparation of plant extracts
All the three plant leaves (Bauhinia Acuminata, Gracinia gummi-gutta L., Cynodon dactylon L.) were washed several times with de-ionized water. 20 g of thoroughly washed leaves were finely cut into small pieces. 10 g of the cut leaves were weighed and added to 150 mL of double distilled water taken in a conical flask and kept under magnetic stirring at 650 rpm for 24 hours. Aqueous extract was separated out using a Whatmann No1 filter paper. The filtrate was collected and stored at 4oC for further use.
Phytochemical screening
Phytochemical screening of leaf extract (Bauhinia acuminata, Gracinia gummi-gutta L., Cynodon dactylon L.) was done to assess qualitatively the presence of various phytochemical constituents [23-25]. The various tests done are given in Table 1.
Table 1 Phytochemical screening tests (qualitative) for Aqueous green leaf extract of (a) Bauhinia acuminata, (b) Cynodon dactylon L., and (c) Garcinia gummi-gutta L.
|
No. |
Component |
Test Name |
Test |
Inference |
Cynodon Dactylon |
Bauhinia Acuminata |
Garcinia Gummi-Gutta |
|
1 |
Alkaloids |
Wagner’s Test |
Filtrates were treated with Wagner’s reagent (Iodine in Potassium Iodide). |
Brown/reddish precipitate |
++ |
++ |
-- |
|
2 |
Carbohydrates |
Benedict’s Test |
Filtrates were treated with Benedict’s reagent and heated gently. |
Orange red precipitate |
- - |
++ |
++ |
|
3 |
Fehling’s Test |
Filtrates were hydrolyzed with dil. HCl, neutralized with alkali and heated with Fehling’s A & B solutions. |
Red precipitate |
- |
++ |
- - |
|
|
4 |
Saponins |
Froth Test |
Extracts were diluted with distilled water to 20 mL and this was shaken in a graduated cylinder for 15 minutes. |
1 cm layer of foam |
++ |
-- |
- - |
|
5 |
Foam Test |
0.5 gm of extract was shaken with 2 mL of water. |
If foam produced persists after shaking for 10 minutes. |
++ |
-- |
- - |
|
|
6 |
Tanins |
Gelatin Test |
To the extract, 1% gelatin solution containing sodium chloride was added. |
White precipitate |
++ |
- - |
- - |
|
7 |
Lead acetate Test |
Small quantities of the extracts were taken separately in water and tested for the presence of tannins with 10% lead acetate solution. |
White precipitate
|
++ |
- - |
++ |
|
|
8 |
Phenols |
FeCl3 Test |
Extracts were treated with 3-4 drops of ferric chloride solution. |
Bluish black colour |
++ |
++ |
- - |
|
9 |
Flavonoids |
Alkaline Reagent Test |
1 mL of filtrate and add 2 mL of dilute NaOH. |
Blue to violet colour (anthocyanins), yellow colour (flavones), yellow to orange (flavonones) |
++ |
++ |
++ |
|
10 |
Lead Acetate Test (Phenolic Flavonoids) |
1 mL of filtrate add 2 mL of 10% lead acetate. |
Yellow precipitate (Flavones) |
++ |
- - |
++ |
|
|
11 |
Proteins |
Xanthoproteic Test |
The extracts were treated with few drops of conc. Nitric acid. |
Yellow colour |
++ |
++ |
++ |
|
12 |
Amino acids |
Ninhydrin Test |
To the extract, 0.25% w/v ninhydrin reagent was added and boiled for few minutes. |
Blue colour
|
+- |
++ |
- - |
|
13 |
Diterpenes |
Copper Acetate Test |
Extracts were dissolved in water and treated with 3-4 drops of copper acetate solution. |
Emerald green colour |
-+ |
++ |
++ |
|
14 |
Cardiac Glycosides |
Keller Killiani Test |
To 0.5 g of extract diluted to 5 mL in water was added 2 mL of glacial acetic acid containing one drop of ferric chloride solution. This was underlayed with 1 mL of concentrated sulphuric acid. |
A brown ring at the interface indicated the presence of a deoxysugar characteristic of cardenolides. A violet ring may appear below the brown ring, while in the acetic acid layer a greenish ring may form just above the brown ring and gradually spread throughout this layer. |
- - |
- - |
- - |
Synthesis of silver nanoparticles
Room temperature method
0.0001 M silver nitrate solution was prepared and taken in a conical flask. 25 mL plant extract (Bauhinia acuminata, Gracinia gummi-gutta L., Cynodon dactylon L.) was added dropwise constantly to 10 mL of AgNO3 solution. Solution was kept on magnetic stirring at 200 rpm for 30 minutes – 2 hours.
AgNO3 → Ag+ + NO3-
Ag+ + 2H2O → Ag0 + 4H+ + O2
The reduction process was complete when the solution turned brownish-black which confirmed the presence of nanoparticles.
Direct heating method
0.0001 M silver nitrate solution was prepared and taken in a conical flask. 25 mL plant extract (Bauhinia acuminata, Gracinia gummi-gutta L., Cynodon dactylon L.) was added dropwise constantly to 10 mL of AgNO3 solution and heating at 60 oC. Solution was kept on magnetic stirring at 200 rpm for 30 minutes. The reduction process was complete when the solution turned brownish-black which confirmed the presence of nanoparticles.
Characterisation of the synthesised silver nanoparticles
Visual observation and standardisation
The bioreduction of silver ions was observed by the colour changes from pale green to pale yellow and then to dark brown. The periodic monitoring of the variation of absorption was done using the calorimetry.
Uv-vis spectroscopy
The UV-Vis spectra of the solution were studied using UV-Vis spectrophotometer. UV-Vis spectroscopy measurements of synthesized silver nanoparticles were characterized by Perkin Elmer UV-Vis spectrophotometer. The scanning range of the samples was 200 -800 nm at a resolution of 1 nm.
Particle size analysis using dynamic light scattering (DLS) technique
In order to find out the particle size distribution the Ag nanoparticle dispersed in water was studied and the data on particle size were extracted using a Zetasizer Nicomp, 390ZLS (Nicomp, USA) using DLS method.
Transmission electron microscopy (TEM)
Samples for TEM analysis were prepared by placing a drop of the silver nanoparticle solution on the TEM copper grid and were dried. The analysis was done using Philips CM200 at operating voltages of 20-200 KV and a resolution of 2.4 ![]() . The TEM measurements were performed and the morphology of the AgNPs was obtained from the micrographs.
. The TEM measurements were performed and the morphology of the AgNPs was obtained from the micrographs.
Antimicrobial activity and minimal inhibitory concentration (MIC)
The antimicrobial activity of AgNPs was evaluated against Gram positive; Staphylococcus aureus, Gram negative; Escherichia coli, Pseudomonas aureginosa by agar well diffusion assay method. 24-hour old cultures were prepared in nutrient broth and the optical density, OD, was measured based on the turbidity measurement to confirm the lag phase stage while inoculation. Approximately 25 mL of the Muller Hinton Agar was poured in the sterilized Petri dishes and plates were prepared. Two replicas of the respective microorganisms were prepared by spreading 100 µL of revived culture on the agar plates with the help of a spreader. Well were bored into it and labeled and loaded with various concentrations Silver nano liquid and incubated for 24 hours at 37 oC. The plates were then examined for the confirmation of zone of inhibitions (ZOI) which became visible as the clear area around the wells. The diameters of these ZOI were measured using a meter scale and the mean value for each organism was recorded in millimeters. The lowest concentration of the AgNPs at which the isolate was completely inhibited (absence of any visible bacterial growth) was recorded as the minimal inhibitory concentration or MIC.
Results and Discussions
Standardisation
Fig. 2 (a) and (b) shows the variation of OD measured at 440 nm for AgNPs synthesized from Bauhinia acuminata, Gracinia gummi-gutta L., Cynodon dactylon L. using heating and without heating methods. It is very clear from the figures that maximum AgNPs which were formed due to heating method and also from the leaf extract of Gracinia cambogia. OD measurements also showed that the maximum AgNPs formed were stable at around 30 minutes for heating method. Hence AgNPs synthesized from heating method was used for comparison.
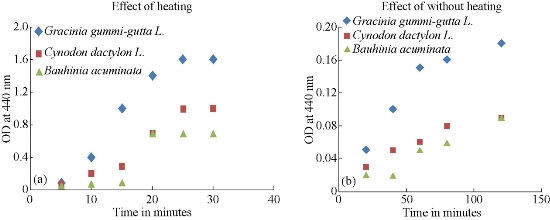
Fig. 2 (a) Effect of heating and (b) without heating on the OD (measured at 440 nm) of the AgNPs, synthesized from different plant extracts.
UV-Vis analysis of AgNPs
Figs. 3, 4 & 5 show the UV-Vis absorption spectra of the various plant extract and the AgNPs synthesized from their leaf extracts by heating methd. Table 2 gives the absorption maxima of the AgNPs synthesized from the various extracts. It clearly shows the formation of AgNPs.
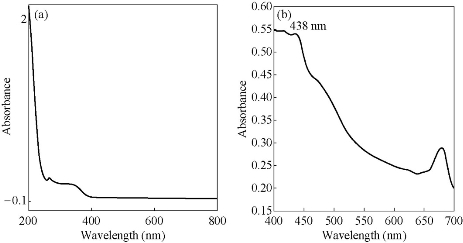
Fig. 3 UV-Visible absorption spectra of (a) green leaf extract of Gracinia gummi-gutta L. and (b) the AgNPs synthesized with heating.
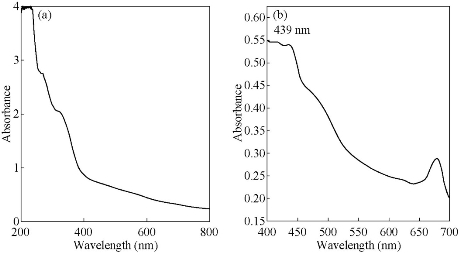
Fig. 4 UV-Visible absorption spectra of (a) green leaf extract of Cynodon dactylon and (b) the AgNPs synthesized with heating.
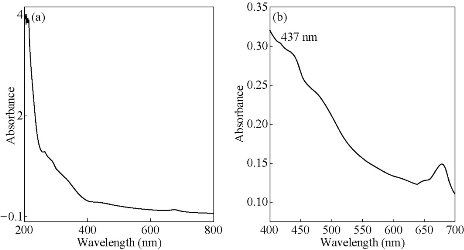
Fig. 5 UV-Visible absorption spectra of (a) green leaf extract of Bauhinia acuminata and (b) the AgNPs synthesized with heating.
Table 2 Maximum absorption (nm) for AgNP’s synthesized from fresh leaf extracts.
|
Ag NP’s synthesized from |
Maximum absorption (nm) |
|
Gracinia gummi-gutta L. |
438 |
|
Cynodon dactylon L. |
439 |
|
Bauhinia acuminata |
437 |
Particle size determination – DLS analysis
The particle size distribution for AgNPs synthesized from various plants by heating method is given in Figs. 6, 7, 8 & 9. Their hydrodynamic diameters were in the range of 100 nm. The AgNPs from Gracinia gummi-gutta L. leaf extract using heating method had minimal diameter.
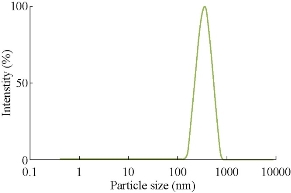
Fig. 6 Particle size analysis of silver nanoparticles synthesized from Gracinia gummi-gutta L. without heating leaf extract.
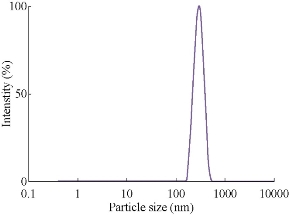
Fig. 7 Particle size analysis of silver nanoparticles synthesized from Gracinia gummi-gutta L. with heating leaf extract.
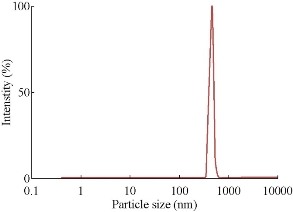
Fig. 8 Particle size analysis of silver nanoparticles synthesized from Cynodon dactylon leaf extract with heating.
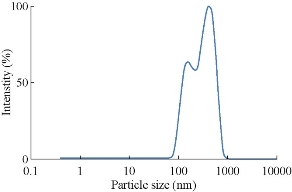
Fig. 9 Particle size analysis of Silver nanoparticles synthesized from Bauhinia acuminata leaf extract with heating.
TEM analysis of AgNPs
TEM imaging was carried out to visualize the shape, size and dispersity of the nanoparticles. The TEM image as in Fig. 10 (b) shows that the synthesized AgNPs from fresh leaf extract of Gracinia gummi-gutta L. with heating were found to be monodispersed, spherical in shape with sizes <10 nm.
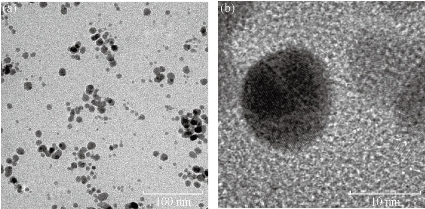
Fig. 10 TEM images of AgNP’s synthesized from the fresh leaf extract of Gracinia gummi-gutta L. in two different magnifications
Antimicrobial activity
Fig. 11 shows the different zones of inhibition for AgNPs against Gram negative bacteria’s P. aureginosa. Controlled experiment with only leaf extracts (blank) does not show any zone of inhibition indicating no antimicrobial activity by the leaf extracts. The AgNPs showed pronounced antimicrobial activity against both types of bacteria. It can be seen from Figs. 12-14 that the AgNPs showed higher zone of inhibition of S. aureus and P.aureginosa than for E.coli. Fig. 15 shows a comparative study of % inhibition of silver nanoparticles from various plant extracts with the standard drug Amikacin. It can therefore be suggested that the accumulation and absorption of AgNPs on the cell walls of the microbes was more in S. aureus and P. aureginosa.
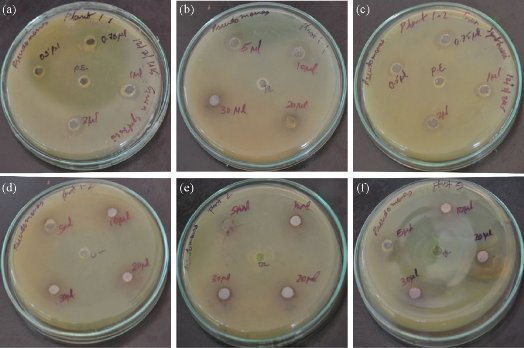
Fig. 11 Agar plates showing the zone of inhibitions against the human pathogen Pseudomonas aureginosa using various concentrations (0.5 μL, 0.75 μL, 1 μL, 2 μL, 5 μL, 10 μL, 20 μL, 30 μL) of AgNP’s synthesized from (a, b) Garcinia gummi-gutta L. without heating the extract, (c, d) Garcinia gummi-gutta L. with heating the extract, (e) Cynodon dactylon L., and (f) Bauhinia Acuminata.
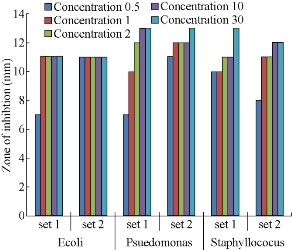
Fig. 12 A comparative study of the antimicrobial activity of various concentrations concentrations (0.5 μL, 1 μL, 2 μL, 10 μL, 30 μL) of AgNPs synthesized from Garcinia gummi-gutta L. without heating the extract (set 1) and with heating the extract (set 2) against pathogenic bacteria E. Coli, Pseudomonas aureginosa, Staphylococcus aureus strains.
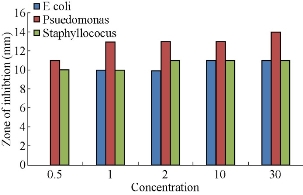
Fig. 13 A comparative study of the antimicrobial activity of various concentrations (0.5 μL, 1 μL, 2 μL, 10 μL, 30 μL) of AgNPs synthesized from Cynodon dactylon green extract against pathogenic bacteria E. Coli, Pseudomonas aureginosa, Staphylococcus aureus strains.
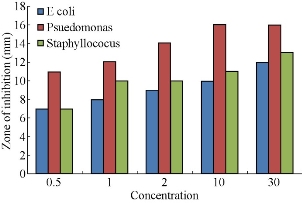
Fig. 14 A comparative study of the antimicrobial activity of various concentrations (0.5 μL, 1 μL, 2 μL, 10 μL, 30 μL) of AgNPs synthesized from Bauhinia acuminata leaf extract against pathogenic bacteria E.Coli, Pseudomonas aureginosa, Staphylococcus aureus strains.
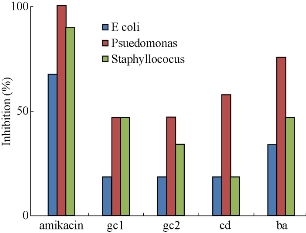
Fig. 15 A comparative study of % inhibition of 30 μL of AgNPs synthesized from leaf extracts of Garcinia gummi-gutta L. without heating the extract (gc1) and with heating the extract (gc 2), Cynodon dactylon (cd) and Bauhinia acuminata (ba) and standard Amikacin against pathogenic bacteria E.Coli, Pseudomonas aureginosa, Staphylococcus aureus strains.
Minimum inhibitory concentrations
The minimum inhibitory concentration for the AgNP derived from different plants is given in Fig. 16. It is clear that the AgNP’s derived by heating method from the leaves of Gracinia gummi-gutta L. were showing pronounced inhibition at even 0.5 μL concentration.
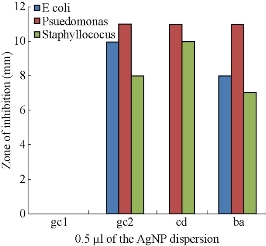
Fig. 16 Comparison of the ZOI’s of AgNP’s derived from leaves of Garcinia gummi-gutta L. without heating the extract (gc1) and with heating the extract (gc 2), Cynodon dactylon (cd) and Bauhinia acuminata (ba) at 0.5 μL concentration against pathogenic bacteria like E. Coli, Psuedomonas auerginosa and Staphyllococus aureus strains.
Conclusions
The current study gives a comparison between the biosynthesis of AgNPs by using the aqueous extracts of the fresh leaves of Garcinia gummi-gutta L., Cynodon dactylon L. and Bauhinia acuminata. The plant extract acts both as a reducing and a capping agents, giving rise to spherical AgNPs. The biosynthesized nanoparticles were characterized by UV-Vis spectroscopy, DLS analysis and TEM. AgNPs were crystalline in nature; the size of the silver nanoparticles was in the range of 20 nm, and their hydrodynamic sizes were around 100 nm. The biosynthesized AgNPs had antibacterial activity and are probable candidates for medical applications, especially in drug delivery process.
Acknowledgements
The authors would like to thank the operators and staff of Amrita Centre for Nanoscience and Nanomedicine, Kochi for the characterisation facility provided there. The authors also like to thank Dr. Bipin Nair, Dean and the staff of Amrita school of Biotechnology, Amritapuri and also would like to mention the BRITE initiatives at Amrita School of Biotechnology, Amritapuri for which this project was considered.
References
Copyright© 2016 Asha R. Pai, Anantha M. Pillai, Abhijith Jayapraksh, and Ajith John. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.