Review
Advances in the Toxicity of Nanomaterials
Xuan Dai, Daxiang Cui*
Department of Bio-Nano-Science and Engineering, Key Laboratory for Thin Film and Microfabrication of Ministry of Education, Institute of Micro- Nano Science and Technology, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, China.
* Corresponding author. Email: dxcui@sjtu.edu.cn
Abstract
Over the past few years, nanomaterials toxicology has emerged as a new exciting field in which theoretical and experimental studies of toxicity of nanomaterials have become a focus, and the importance of carbon nanotubes (CNTs), graphene oxide (GO), quantum dots (QDs), magnetic nanoparticles (MNPs), and amorphous silica nanoparticles (ASN) as special nanomaterials to the fundamental development in biomedical engineering has begun to be recognized. In particular, interaction between nanomaterials and nucleic acids, proteins or cells, animals, environment, etc. has become a new interdisciplinary frontier, there is an increasing need for a more systematic study of the basic issues involved in nanomaterials toxicity and potential toxicity-reducing methods, great advances have been and are being made in nanomaterials’ biological effects and application in disease diagnosis and therapy. Here we review some of the main advances in this field over past few years, and discuss about the concepts, issues, approaches, and challenges, with the aim of stimulating a broader interest in studying the toxicological mechanism of nanomaterials and potential toxicity- eliminating measurement.
Keywords: Amorphous silica nanoparticle, Carbon nanotube, Graphene oxide, Quantum dot, Magnetic nanoparticle, Nanotoxicity
Citation: X. Dai and D.X. Cui, Advances in the Toxicity of Nanomaterials Nano Biomed. Eng. 2012, 4(3), 150-156.
DOI: 10.5101/nbe.v4i3.p150-156.
1. Introduction
Nanotechnologies have been growing rapidly during the past decade, giving rise to an expanding discipline representing an innovative leap that, according to numerous scientists, will likely revolutionize the global industry. The area of nanotechnology has expanded so much that nowadays it is difficult to categorize them. A few widely recognized important areas include nanofabrication and nano-electro-mechanical systems (NEMS) [1,2], nanoscale chemical and biological sensors [3-5], single-molecule science and imaging technologies [6], self-assembly and nanodevices [7-9], nanobiology and nanomedicine [10-12], and production and characterization of nanomaterials [13]. Nanotechnology is not as much a discipline, like chemistry or physics, as a tool for manipulating matter as its finest scale. Nanoscale is usually defined as smaller than a one tenth of a micrometer in at least one dimension, though this term is sometimes also used for materials smaller than one micrometer. At this size, materials may exhibit unique properties compared with bulk materials, including altered color, magnetism, optical properties, increased strength, flexibility, or reactivity, and improved electrical conductivity or absorption, etc. As a result, engineered nanomaterials have become the focus of extensive research in diverse areas, including electronics, materials engineering, energy production and conservation, and biomedical applications. However, the same properties that make nanomaterials desirable in these various applications have the potential to alter the biological properties that impact human healthcare, nature and the environment, and climate. The little data now available highly suggest that some nanomaterials show signs of toxicity [14], being mobile or persistent in the environment [15], or affecting microorganisms [16]. The data highlights the fact that we know little about the environmental and health effects of engineered nanomaterials, especially the real risks generated by the consequences of interaction between the novel classes of nanomaterials being produced and the human body or environment. These risks and their corresponding uncertainties highly suggest that nanotechnology should be regarded as a double-edged sword. The risks of nanomaterials should be identified before they are incorporated into products for commercial production. Nanotoxicology is a sub-specialty of toxicology to deal with the study and application of toxicity of nanomaterials. Because of quantum size effects and large surface area to volume ratio, nanomaterials have unique properties compared with their larger counterparts. The understanding of the safety, environmental and human health implications of nanomaterials and products has become a focus worldwide. Nanotoxicological studies are intended to determine whether and to what extent these properties may pose a threat to the environment and to human beings. Up to date, it is still a great challenge for the assessment of the safety of nanotechnology-based products and to encourage nanotechnological advances that can address the needs of citizens and contribute to sustainable development objectives. Safety evaluation of nanomaterials should be done from five scales, that is, molecular level, cell level, animal level, human body level, and environment and climate level. In fact, the nanomaterials are how to affect human body, plants, and environment as well climate has become very key challengeable problems. Up to date, no data fully shows that the procedure and mechanism of nanomaterials interacting with human body or healthcare. Here we review some of the main advances in the toxicity of common nanomaterials such as carbon nanotubes, grapheme oxides, fluorescent magnetic nanoparticles, and amorphous silica nanoparticles over past few years, and discuss about the concepts, issues, approaches, and challenges, with the aim of stimulating a broader interest in studying the toxicological mechanism of nanomaterials and potential toxicity-eliminating measurement.
2. Advances of nanomateirals toxicity
2.1 Advances of CNTs toxicity
CNTs including single walled carbon nanotubes (SWCNTs) and multi walled carbon nanotubes (MWCNTs), as a class of stiff, stable and hollow nanomaterials with many unique properties such as mechanical, physical and chemical properties, have recently emerged as a new option for possible use in methodologies of cancer treatment, bioengineering, and gene therapy. For example, CNTs have used as atomic force microscopy (AFM) tip to obtain atomic-resolution imaging of biological molecules such as DNA and proteins [17,18]. CNTs can be filled with DNA or peptide molecules and have highly potential in gene or peptide storage and delivery system for molecular therapy of diseases [19,20]. The application of CNTs in biomedical engineering is highly depend upon their biocompatibility and toxicity. Up to date, CNTs showed sign of toxicity. For example, SWCNTs can inhibit the growth of human fibroblast cells and HEK293 cells [21], decrease the adhesive ability of cells, and induce cell apoptosis in a dose- and time-dependent manner as shown in Fig. 1. SWCNTs are about 1 nm in diameter and several microns in length, and often pack tightly together to form rods or ropes of microscopic size. Unprocessed nanotubes are very light and could become airborne and potentially reach the lungs. No-purified CNTs could not induce signifcant signs of lung toxicity four weeks after a single intratracheal administration in guinea pigs [22]. It is also reported that CNTs could induce acute inflammatory pulmonary effects in mice [23-25], for example, epithelioid granulomas and interstitial infammation in the lungs of mice. SWCNTs can influence the growth of natural plants [26]. For example, SWCNTs exhibited dual-phase regulation to Arabidopsis mesophyll cells, under the dose of 50 μg mL−1 SWCNTs in medium, SWCNTs stimulate plant cells to grow out trichome clusters on their surface, with more than 50 μg mL−1SWCNTs in medium, SWCNTs exhibited obvious toxic effects to the protoplasts such as increasing generation of ROS, inducing changes of protoplast morphology, changing green leaves into yellow, and inducing plant cells' necrosis and apoptosis, as shown in Fig. 2. We also observed that generation 5 polyamidoamine dendrimer-functionalized multi-walled carbon nanotubes could enter into embryonic stem (ES) cells quickly, more than 20 μg mL−1dose caused ES cells become smaller and smaller as the incubation time increased, and inhibited cell growth in dose- and time-dependent means, less than 5 μg mL−1dose improves ES differentiation [27].
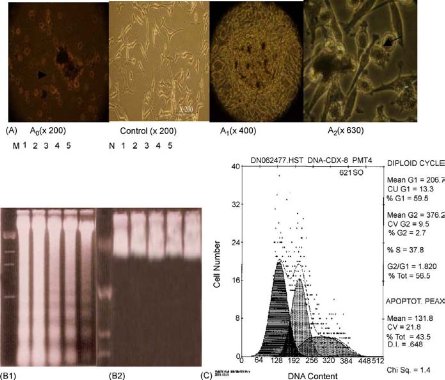
Fig. 1 Apoptosis of HEK293 cells induced by SWCNTs. A: morphological changes of HEK293 cells cultured with 25 μg mL−1 SWCNTs for three days; A0: showing cells become round and floating with apoptotic characteristics; control: showing normal morphological cells; A1: showing nodular structures composed of SWCNTs and apoptotic cells; A2: showing apoptotic cells attached by SWCNTs. B1: DNA electrophoresis of cells cultured with 25 μg mL−1 SWCNTs for 1-5 days, M molecular marker, no. 1-5 denote the results of cells cultured for day 1-5, respectively; B2: DNA electrophoresis results of control cells cultured for day 1-5; C: the cell cycle distribution of HEK293 cells cultured with 25 μg mL−1 SWCNTs for four days, the percentage of sub-G1 cells was 43.5%. Copyright permission from ref. 22.
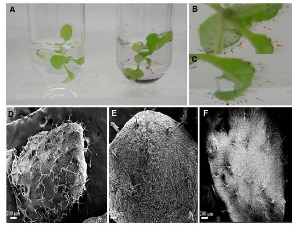
Fig. 2 Optical images and SEM images of Arabidopsis mesophyll cells exposed to SWCNTs. (A) Plant cells treated without or with 15 and 25 μg mL−1 SWCNTs; (B) and (C) plant cells treated with 15 and 25 μg mL−1 of SWCNTs for 24 h. (D) Plant cells treated with 25 μg mL−1 of SWCNTs for 48 h; (E) plant cells without exposed SWCNTs as control; (F) plant cells treated with 15 μg mL−1 of SWCNTs for 24 h. Copyright permission from ref. 26.
2.2 Advances of graphene oxide toxicity
Graphene oxide (GO) is one of the most important graphene derivatives and has been extensively studied in recent years. Owing to its small size, intrinsic optical properties, large specific area, low cost, and useful non- covalent interactions with aromatic drug molecules, GO is the potential candidates for biomedical applications [28-30]. Up to date, GO showed the sign of toxicity. For example, Chang, et al. [31] evaluated the morphology, viability, mortality and membrane integrity of A549 after exposure to GO, results showed that GO could hardly enter cells and no obvious toxicity to A549 cells. But GO induces the cellular oxidative stress even at low concentration and induce a slight decrease of the cell viability at high concentration. Very similar results were reported by Ryoo,et al. [32], showing that GO was highly biocompatible and improved gene transfection efficacy in NIH-3T3 fibroblasts. But, Wang, et al. [33] reported that GO exhibits dose- dependent toxicity to cells and animals, and cannot be cleaned by kidney, inducing lung inflammatory lesions, as shown in Fig. 3 and Fig. 4. Biris, et al. [34] also reported that both GO and CNTs induce cytotoxic effects on PC-12 cells, considering that CNTs are more toxic than graphene and that the shape of these carbon-based nanomaterials plays an important role in their cytotoxicity.
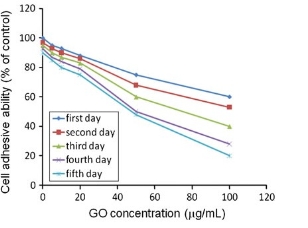
Fig. 3 GO-treated HDF cell adhesion ability measured by centrifugation method. The percentage of adhesive cells decreased with the increase in GO concentration and culture time. Copyright permission from ref. 34.
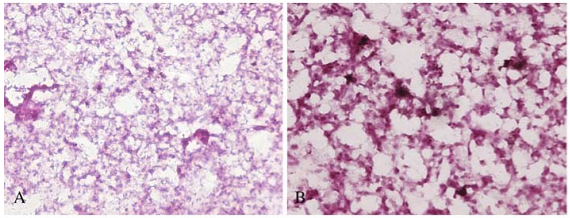
Fig. 4 The light micrograph of lung tissues from mice exposed to GO of 0.1 mg at different exposure time: (A) 7 days, (B) 30 days. (magnification: X200). Copyright permission from ref. 33.
2.3 Advances of quantum dots toxicity
Marked cytotoxicity to PC12 and N9 cells at low concentrations, but pre-treatment of cells with the antioxidant N-acetylcysteine and with bovine serum albumin could significantly reduce the QD-induced cell death, as shown in Fig. 5. Derfus, et al. [39] demonstrated that CdSe-core QDs were toxic under certain conditions using primary hepatocytes as a liver model. Current data further suggests that cytotoxicity of QDs correlates with the 2+ Quantum dots (QDs) are nanoscale semiconductor liberation of free Cd ions due to deterioration of the crystals composed of groups II-VI or III-V elements, and are defined as particles with physical dimensions smaller than the exciton Bohr radius [35]. When a photon of visible light hits such a semiconductor, some of their electrons are excited into higher energy states. When they return to their ground state, a photon of a frequency characteristic of that material is emitted. QDs are emerging as alternative or complementary tools to the organic florescent dyes which can be exploited for cellular imaging, immunoassays, DNA hybridization, and optical barcoding [36]. Therefore, QDs provide a new functional platform for bio-analytical sciences and biomedical engineering. However, QDs’ toxicity affects their in vivo application. For example, several studies showed that uncapped QDs could induce damage to mammalian cells through the generation of free radicals and DNA damage CdSe lattice. When appropriately coated, CdSe-core QDs can be rendered nontoxic and used to track cell migration and reorganization in vitro. Hoshino, et al. [40] revealed the toxicity of QDs in biological systems is not dependent on the nanocrystal particle itself but on the surface molecules. In the case of QDs, surface processing of QDs could overcome the toxicity of nanomaterials. For example, surface modifications with polyethylene glycol (PEG) or car- boxylic acids, and encapsulation in polymeric micelles can improve the stability and biocompatibility of the QD core/shell complex.
2.4 Advances of magnetic nanoparticles toxicity
In general, magnetic nanoparticles (MNPs) are constituted by magnetite (Fe O). They have an external resulting in different types of cell death, including cell apoptosis [37].Lovric, et al. [38] found that CdTe QDs exhibited surface covered by a stabilizing or surfactant agent to avoid the spontaneous clotting induced by the interaction of the magnetic dipoles. MNPs are widely studied and applied in biology and medicine, magnetic resonance imaging, diagnostics, immunoassays, RNA and DNA purification, gene cloning, and cell separation and purification [41-43]. These MNPs are generally exist in a core-shell configuration in which biological species such as cells, nucleic acids, and proteins are bound to the magnetic core through organic linkers, which are often organized as a polymeric shell around the core [44]. MNPs offer some attractive possibilities in biomedicine. First, they have controllable sizes ranging from a few nanometers up to tens of nanometers, which mean that they can get close to a biological entity of interest. Second, the nanoparticles are magnetic, which means that they obey Coulmb’s law, and can be manipulated by an external magnetic field gradient. Third, the magnetic nanoparticles can be made to resonantly respond to a time-varying magnetic field, with advantageous results related to the transfer of energy from the exciting field to the nanoparticle. Kim [45] tested the toxicity and tissue distribution of magnetic nanoparticle in mice. They synthesized silica-overcoated MNPs containing rhodamine B isothiocyanate (RITC) within a silica shell of controllable thickness [MNPs@SiO2 (RITC)]. After intraperitoneal administration of MNPs@SiO2 (RITC) for 4 weeks into mice, the nanoparticles were detected in the brain, indicating they can penetrate blood-brain barrier (BBB) and be present in various organs without causing apparent toxicity. They concluded that magnetic nanoparticles of 50 nm size did not cause apparent toxicity. Jain [46] tested the bio-distribution and biocompati- bility of oleic acid (OA)-Pluronic-coated iron oxide MNPs in rats. They analyzed the changes in serum and tissue iron levels over 3 weeks after intravenous administration and found serum iron levels gradually increased for up to 1week but levels slowly declined thereafter. Bio-distribution of iron in various body tissues changed with time but greater fraction of the injected iron localized in the liver and spleen. The increase in oxidative stress was tissue dependent, reaching a peak at ~3 days and then slowly declining thereafter. So they concluded that MNPs can be safely used for drug delivery and imaging applications. We also prepared hydrophilic high-luminescent magnetic nanocomposites (LMNCs), and investigated their influence on HEK293 cells and mice [47]. Results showed that the water soluble FMSNPs with dose less than 50 μg mL−1 exhibited well biosafety to HEK293 cells, FMSNPs with low dose (0.1 mg mL−1) and middle dose (0.3 mg mL−1) did not exhibit toxicity to mice, as shown in Fig. 6. LMNCs exhibited good biocompatibility to cells and animals under the dose of 50 μg mL−1, and own great potential biomedical applications.
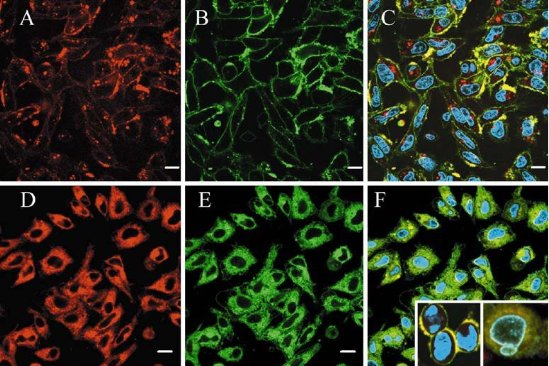
Fig. 5 Confocal micrographs of PC12 cells treated with positively charged, red QDs. A-C PC12 cells were incubated with red cationic QD (3.75 μg mL−1) for 5 min (A), with DAF (1 μM) for 1 min (B) and the obtained images were superimposed (C). D-F PC12 cells incubated with high concentration of QDs (37.5 μg mL−1) for 5 min (D), with DAF (1 μM) for 1 min (E), Hoechst (10 μM) for 1h and the obtained images were superimposed (F). Scale bar 10 μm. Inserts show rounding of cells and nuclear blebbing after 1 h treatment with QDs (37.5 μg mL−1). Copyright permission from ref.38.
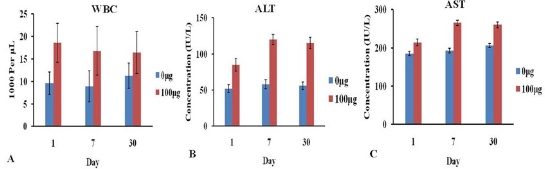
Fig. 6 Hematology and biochemical markers of mice after intravenous injection FMNPs: (A) WBC. (B) ALT. (C) AST. Copyright permission from ref. 47.
2.5 Advance of amorphous silica nanoparticles toxicity
Amorphous silica nanoparticles (ASNs) are incr- easingly used in diagnostic and biomedical application due to its ease of production and relatively low cost. It is generally regarded as safe, and has been approved for use as a food or animal feed ingredient. Some literatures revealed that ASNs may present toxicity concerns at high doses. Lin, et al. [48] investigated the cytotoxicity of silica nanoparticles in human lung cancer cells and results in a dose-dependent cytotoxicity. Yu,et al. [49] examined the uptake, localization, and the cytotoxic effects of well-dispersed ASNs in mouse keratinocytes (HEL-30), as shown in Fig. 7.Mouse keratinocytes were exposed for 24 h to various concentrations of amorphous silica nanoparticles in homogeneous suspensions of average size distribution (30, 48, 118, and 535 nm SiO2) and then assessed for uptake and biochemical changes. Results of transmission electron microscopy reveals all sizes of silica were taken up into the cells and localized into the cytoplasm. The LDH assay shows LDH leakage was dose- and size-dependent with exposure to 30 and 48nm nanoparticles. However, no LDH leakage was observed for either 118 or 535 nm nanoparticles. They concluded that size of particles is critical to produce biological effects.
In anticipation of potential human exposure to silica, Chang, et al. [50] investigated the response of several normal fibroblasts to varying doses of ASNs or composite nanoparticles of silica and chitosan. A cell proliferation assay indicates that silica nanoparticles are nontoxic at low dosages but that cell viability decreases at high dosages. A LDH assay indicates that high dosages of silica induce cell membrane damage. In contrast, silica- chitosan composite nanoparticles induce less inhibition in cell proliferation and less membrane damage. This study suggests that the cytotoxicity of silica to human cells depends strongly on their doses and it could be significantly reduced by synthesizing silica with chitosan.
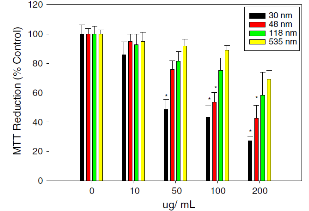
Fig. 7 SEffect of silica nanoparticles on MTT reduction (% control). The HEL-30 cells were dosed with different sizes (30, 48, 118 and 535 nm) and various concentrations of silica (0, 10, 50, 100 and 200 μg mL−1) for 24 h. Size- and dose-dependent MTT reduction in 30 and 48 nm at higher concentrations (200 μg mL−1) produced significant toxicity when compared to large sizes (118 and 535 nm). Three independent experiments (n=4) were carried out, and data are means±SD. *Significantly different from control at p<0.05. Copyright permission from ref. 48.
3. Some Major Challenges in Nanotoxicity
Nanotoxicity studies, like any emerging field, face many challenges. Up to date, the effects of nanoparticles on cell proliferation and differentiation as well mice were reported for many times. However, few reports are closely associated with the influences of nanomaterials on human body, natural plants, air, environment and earth climates, how to clarify the mechanism of interaction between nanomaterials and human, natural environment and climates is a great challenge. To date, we still lack good experimental and theoretical methods to investigate, especially quantitatively, many problems in nanomaterials structural, mechanical, and dymanic properties, it is still a great challenge to fully quantify the model of interaction between nanomaterials and cells, etc.
4. Technological Prospects of Nanotoxicity
Up to date, although toxicity studies of nanomaterials have obtained some data, for example, inducing cell death and inflammation, however, their toxicity mechanism is still not clarified well, toxicity studies just begin. From now on, we should strengthen the research of the following aspects of nanomaterials: First, due to the big difference between the vitro and vivo environment, we should strengthen the experiments in vivo and further confirm the reliability of the results from in vitro experiments. Secondly, we should get a better understanding of the transfer and accumulation of nanoparticles in the human body through possible absorption and clinical administrations, as well as the release mechanism and location of the heavy metal content. Finally, it should increase monitoring of the concentration of nanoparticles and establish relevant regulations to manage and regulate the production operation. In order to predict and assess the impact of nanomaterials on living organisms scientifically, we need more systematic and long-term study. Whether foreign or domestic, nanomaterials and nanotechnology research on human health has just started, it needs the joint efforts on biotechnology, nanotechnology, medicine, chemical and physical means. While the governments and scientists should be committed to the development and research of non-toxic nano-materials to reduce the toxicity of nanomaterial. We believe that the nanomaterials will make great contribution to human beings in near future.
References
1 Tambe NS, Bhushan B. Micro/nanotribological characterization of PDMS and PMMA used for BioMEMS/NEMS applications. Ultra- microscopy., 2005; 105:238-247. doi:10.1016/j.ultramic.2005.06.050
2 Burton Z, Bhushan B. Hydrophobicity, adhesion, and friction pro- perties of nanopatterned polymers and scale dependence for micro- and nanoelectromechanical systems. Nano Lett., 2005; 5:1607-1613. doi:10.1021/nl050861b
3 Park SJ, Taton TA, Mirkin CA. Array-based electrical detection of DNA with nanoparticle probes. Science, 2002; 295: 1503-1506.doi: 10.1126/science.1067003
4 Drummond TG, Hill MG, Barton JK. Electrochemical DNA sensors. Nat. biotechnol., 2003; 21: 1192-1199. doi:10.1038/nbt873
5 Dubertret B, Calame M, Libchaber AJ. Single-mismatch detection using gold-quenched fluorescent oligonucleotides. Nat. biotechnol., 2001; 19: 365-370. doi:10.1038/86762
6 Attard P, Moody MP, Tyrrell JW. Nanobubbles: the big picture. Phy- sica A, 2002; 314: 696-705. doi:10.1016/S0378-4371(02)01191-3
7 Zhang Z, Fan C, He L. Development of nano-scale DNA computing devices. Cur. Nanosci., 2005; 1: 91. doi:10.2174/1573413052953138
8 Cui Y, Wei Q, Park H, Lieber, CM. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science, 2001; 293: 1289-1292. doi:10.1126/science.1062711
9 Kim P, Lieber CM. Nanotube nanotweezers. Science, 1999; 286: 2148-2150. doi:10.1126/science.286.5447.2148
10 Mirkin CA, Taton TA. Semiconductors meet biology. Nature, 2000; 405: 626-627. doi:10.1038/35015190
11 Bottini M, Bruckner S, Nika K, Bottni N. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol Lett., 2006; 160: 121-126. doi:10.1016/j.toxlet.2005.06.020
12 Byon HR, Hong BJ, Gho YS, Park JW. Pseudo 3D single walled carbon nanotube film for BSA-free protein chips. ChemBioChem., 2005; 6: 1331-1334. doi:10.1002/cbic.200500081
13 Wang X, Zhuang J, Peng Q, Li Y. A general strategy for nanocrystal synthesis. Nature, 2005; 437: 121-124. doi:10.1038/nature03968
14 Inoue K, Takano H, Ohnuki M. Size effects of nanomaterials on lung inflammation and coagulatory disturbance. Int. j. immunopath. ph., 2008; 21: 197. doi:10.1498/289910
15 Klaine SJ, Alvarez PJ, Handy, RD. Nanomaterials in the environ- ment: behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem., 2009; 27: 1825-1851. doi: 10.1897/08-090.1
16 NiaziandManBockGu JH. Toxicity of metallic nanoparticles in microorganisms-a review. Atmospheric and Biological Environ- mental Monitoring., 2009; 193. doi:10.1729/97-810.3
17 Nagao E, Nishijima H, Akita S, Nakayama Y. The cell biological application of carbon nanotube probes for atomic force microscopy: comparative studies of malaria-infected erythrocytes. J. electron microsc., 2000; 49: 453-458. doi:10.1628/82-723.7
18 Hafner JH, Cheung CL, Woolley AT. Structural and functional imaging with carbon nanotube AFM probes. Progress in biophysics and molecular biology., 2001;77: 73-110. doi: 10.1016/S0079-http://nanobe.org 6107(01)00011-6
19 Cui D, Gao H. Advance and prospect of bionanomaterials. Biotechnol. progr. 2003; 19: 683-683. doi:10.1021/bp025791i
20 Cui D, Ozkan CS, Kong Y. Encapsulation of Pt-labelled DNA molecules inside carbon nanotubes. Mech. Chem. Biosyst., 2004; 1: 113-121. doi:10.1557/PROC-820-O4.6
21 Cui D, Tian F, Ozkan CS, Wang M. Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol Lett., 2005; 155: 73-85. doi:10.1016/j.toxlet.2004.08.015
22 Huczko A, Lange H, Calko E, Droszcz P. Physiological testing of carbon nanotubes: are they asbestos-like. Fullerene science and technology., 2001; 9: 251-254. doi:10.1081/FST-100102973
23 Chan HC, Kuo SC, Huang LJ. A phenylacetate derivative, SCK6, inhibits cell proliferation via G1 cell cycle arrest and apoptosis. Eur. j. pharmacol., 2003; 467: 31. doi:10.1016/S0014-2999(03)01596-6
24 Lam CW, James JT, McCluskey R. Pulmonary toxicity of single- wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation.Toxicol Sci.,2004; 77:126-34. doi: 10.1093/toxsci/kfg243
25 Poland CA, Duffin R, Kinloch I, Maynard A. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos- like pathogenicity in a pilot study. Nat. Nanotechnol., 2008; 3: 423- 8. doi:10.1038/nnano.2008.111
26 Yuan HG, Hu SL, Huang P, Song H. Single walled carbon nano- tubes exhibit dual-phase regulation to exposed arabidopsis meso- phyll cells. Nanoscale Res. Lett.,2010. doi: 10.1007/s11671-010- 9799-3
27 Cui D, Zhang H, Wang K, Toru A. Endocytosis of fluorescent car- bon nanotubes in embryonic stem cells. Nanoscale Res., doi: 10. 1007/s11671-009-9292-z
28 Lu CH, Zhu CL, Li J. Using graphene to protect DNA from cleavage during cellular delivery. Chem. Comm., 2010; 46: 3116-3118. doi:10.1039/b926893f
29 Sun, X. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res., 2008; 1:203-212. doi:10.1007/s12274-008-8021-8
30 Zhang L, Xia J, Zhao Q. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small, 2009; 6: 537-544. doi: 10.1002/smll.200901680
31 Chang Y, Yang ST, Liu JH, Dong E. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol Lett., 2011; 200:201-10. doi:10.1016/j.toxlet.2010.11.016
32 Ryoo SR, Kim YK, Kim MH, Min DH.Behaviors of NIH-3T3 fibro- blasts on graphene/carbon nanotubes: proliferation, focal adhesion, and gene transfection studies. ACS Nano, 2010; 4: 6587-6598. doi:10.1021/nn1018279
33 Wang K, Ruan J, Song H, Zhang JL. Biocompatibility of graphene oxide. Nanoscale Res., 2010. doi:10.189/7382-2312.1
34 Zhang Y, Ali SF, Dervishi E. Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cells. ACS Nano, 2010; 4: 3181-3186. doi:10.1021/nn1007176
35 Chan WCW, Maxwell DJ, Gao X. Luminescent quantum dots for multiplexed biological detection and imaging. Curr. Opin. Biotech., 2002; 13: 40-46. doi:10.1016/S0958-1669(02)00282-3
36 Jamieson T, Bakhshi R, Petrova D. Biological applications of quantum dots. Biomaterials., 2007; 28: 4717-32. doi:10.1016/j. biomaterials.2007.07.014
37 Chan, W.H., N.H. Shiao, and P.Z. Lu, CdSe quantum dots induce apoptosis in human neuroblastoma cells via mitochondrial- dependent pathways and inhibition of survival signals. Toxicol Lett., 2006; 167: 191-200. doi:10.1016/j.toxlet.2006.09.007
38 Lovric J, Bazzi HS, Cuie Y. Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. J. Mol. Med. (Berl), 2005; 83: 377-85. doi: 10.1007/s00109-004-0629-x
39 Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett., 2004; 4: 11-18. doi:10. 1021/nl0347334
40 Hoshino A, Fujioka K, Oku T. Physicochemical properties and cellular toxicity of nanocrystal quantum dot depend on their surface modification. Nano Lett., 2004;4:2163-2169.doi:10.1021/nl048715d
41 Wang D, He J, Rosenzweig N. Superparamagnetic Fe2O3 beads- CdSe/ZnS quantum dots core-shell nanocomposite particles for cell separation. Nano Lett.,2004; 4: 409-413. doi:10.1021/nl035010n
42 Wang FH, Yoshitake T, Kim Dk. Determination of conjugationefficiency of antibodies and proteins to the superparamagnetic iron oxide nanoparticles by capillary electrophoresis with laser-induced fluorescence detection. J. Nanopart. Res.,2003; 5: 137-146. doi: 10.1023/A:1024428417660
43 Gupta AK, Curtis ASG. Lactoferrin and ceruloplasmin derivatized superparamagnetic iron oxide nanoparticles for targeting cell sur- face receptors. Biomaterials. 2004; 25: 3029-3040. doi:10.1016/ j.biomaterials.2003.09.095
44 Chomoucka J, Huska D. Magnetic nanoparticles and targeted drug delivering. Pharmacol. res., 2010; 62: 144-149. doi:10.1016/ j.phrs.2010.01.014
45 Kim JS, Yoon TJ, Yu KN, Kim BG. Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicol Sci., 2006; 89: 338-47. doi: 10.1093/toxsci/kfj027
46 Jain TK, Reddy MK, Morales MA. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol. pharmaceut., 2008; 5: 316-327. doi:10.1021/mp7001285
47 Ruan J, Wang K, Song H, Xu X. Biocompatibility of hydrophilic silica-coated CdTe quantum dots and magnetic nanoparticles. Nanoscale Res. Lett., 2011; 6: 299. doi:10.1186/1556-276X-6-299
48 Lin W, Huang YW, Zhou XD, Ma YF. In vitro toxicity of silica anoparticles in human lung cancer cells. Toxicol. Appl. Pharm., 2006; 217: 252-259. doi:10.1016/j.taap.2006.10.004
49 Yu KO, Grabinski CM, Schrand AM. Toxicity of amorphous silica nanoparticles in mouse keratinocytes. J. Nanopart. Res., 2008; 11: 15-24. doi: 10.1007/s11051-008-9417-9
50 Chang Y, Yang ST, Liu JH, Dong E. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett., 2011; 200: 201-210. doi:10.1016/j.toxlet.2010.11.016
Copyright:(c) 2012 X. Dai and D.X. Cui. This is an open- access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.