Article
Dendrimer Modified SWCNTs for High Efficient Delivery and Intracellular Imaging of Survivin SiRNA
Can Wang 1, Zhiming Li 2, Bing Liu 2, Quande Liao 1*, Chenchen Bao 2, Hualin Fu 2, Bifeng Pan 2, Weilin Jin 2, Daxiang Cui 2*
1 Xiangya Hospital of Central South University, 87 Xiangya Road, Changsha410008, Hunan, P. R. China.
2 Institute of Nano Biomedicine and Engineering, Key Laboratory for Thin Film and Microfabrication Technology of the Ministry of Education, Research Institute of Micro/Nano Science and Technology, Key Laboratory for the Genetics of Developmental & Neuropsychiatric Disorders of Ministry of Education, Bio-X Center, Shanghai JiaoTong University, Dongchuan Road 800, 200240 Shanghai, P. R. China.
* Corresponding authors: Quande Liao (qiandeliao@yahoo.cn ); Daxiang Cui (dxcui@sjtu.edu.cn)
Citation: C. Wang et al. Dendrimer modified SWCNTs for High Efficient Delivery and Intracellular Imaging of survivin siRNA. Nano Biomed. Eng. 2013, 5(3), 131-136.
DOI: 10.5101/nbe.v5i3.p131-136.
Abstract
Herein we reported that polyamidoamine dendrimer (PAMAM) modified single walled carbon nanotubes (PSWCNTs) were successfully used for high efficient delivery and intracellular imaging of survivin siRNA vector. No.4 generation of PAMAM dendrimers were used to modify single walled carbon nanotubes, and characterized by high resolution transmission electron microscopy (HR-TEM), and atom force microscopy (AFM), survivin shRNA vectors were constructed and identified, and then dendrimer-modified SWCNTs were mixed with survivin shRNA vectors, resultant dendrimer-SWCNTs-survivin shRNA vector mixtures co-cultured with human gastric cancer MGC803 cells, then the expression of survivin shRNA vectors in MGC803 cells was observed by fluorescent microscopy, cell viability was analyzed by MTT method. Results showed that survivin shRNA vector was successfully expressed in MGC803 cells, and MGC 803 cell growth was markedly inhibited. In conclusion, dendrimer (PAMAM) modified single walled carbon nanotubes can be used for high efficient delivery and intracellular imaging of siRNA, and own great potential in applications such as gene or drug delivery and tumor targeted imaging and simultaneous therapy in near future.
Keywords: Polyamidoamine dendrimer; Single walled carbon nanotube; Delivery system; siRNA; Imaging
Carbon nanotubes, as a class of stiff, stable and hollow nanomaterials with many unique properties such as mechanical, physical and chemical properties, have been being explored application in biomedical engineering and medical chemistry [1-3]. For example, carbon nanotubes have been used as delivery system for gene therapy [4], also used for biosensor for ultrasensitive detection of biomarkers [5], more important, carbon nanotubes can be used for near infrared imaging and photothermal therapy [6]. In our previous work, we also confirmed that carbon nanotubes can be filled with DNA or peptide molecules [7,8], can take antisense oligonucleotides into HL-60 cells and inhibited tumor cell growth [9], highly suggesting that carbon nanotubes may be good delivery system and have highly potential in gene or drug storage and delivery system in molecular therapy of diseases. Up to date, many data showed that carbon nanotubes own cytotoxicity, and single walled carbon nanotubes exhibited stronger toxicity than multi-walled carbon nanotubes (MWCNTs) [10-12]. How to decrease carbon nanotubes’ toxicity and enhance carbon nanotubes biocompatibility have become scientists’ concerns, many methods have been tried [13]. So far, CNTs’ toxicity may arise from resident metal nanoparticles, and induced ROS reaction, therefore, CNTs’ surface functionalization becomes the key step to enhance CNTs’ biocompatibility [14,15]. Our data showed that single walled carbon nanotubes exhibit dual-phase regulation to exposed cells, which is highly depending on SWCNTs’ amount and surface properties. Some studies show that CNTs can be kicked out of mice or other animal bodies by kidney or liver-gall intestinal tracts [16]. Therefore, CNTs exhibited limited toxicity in vivo, and still may own potential applications in human body under the reasonable dose control. In our previous work, we established the method of dendrimer grown on the surface of carbon nanotubes [17], we also used dendrimer modified MWCNTs to realize gene high efficient delivery for tumor therapy or stem cell growth regulation [18-20]. However, few reports is closely associated with use of dendrimer modified single walled carbon nanotubes as siRNA delivery system. RNA interference (RNAi) is the phenomenon that small double-stranded RNA can knock down the expres- sion of complamentary gene sequences [21]. RNAi has been observed in almost all of live things including mammals. It has evolved into a powerful tool to study gene functions. RNAi works by cleaving and destroying its homologous mRNA [22,23]. Activation of shRNA has been achieved via RNA-induced silencing complexes (RISCs) by unwinding the hairpin double RNA strands. The unwound RNA strands subsequently guide the complex to the complementary RNA molecules, where the complex cleaves and destroys the target RNA, which results in RNAi of target mRNA. As the double-stranded RNA bind complementarily to each other as 19-25 base pairs, the small double-stranded RNA is also called as short hairpin RNA (shRNA) [24-27]. In this study, we prepared no.4 generation of polyami- doamine (PAMAM) dendrimer modified single walled carbon nanotubes, and constructed survivin shRNA vector, investigated the efficiency of the dendrimer- modified SWCNTs to deliver survivin siRNA into MGC803 cells, and its biocompatibility, as well as their effects on MGC803 cells, which lay foundation for further investigating dendrimer modified SWCNTs applications in tumor targeted imaging and gene therapy.
2.1 Materials
SWCNTs were purchased from the Shenzhen Nanoport Company. Polyamidoamine (PAMAM) dendrimers were kept in our Lab. Human gastric cancer MGC803 cells were obtained from Shanghai Wuli Biotechnolony. DMEM (Gibco BRL, Gaithersburg, MD, U.S.A.) contains 10% fetal calf serum (FCS, Gibco BRL), penicillin (100 U/ml), streptomycin (100 μg/mL), L-glutamine 2 mM (ICN Biomedicals, Costa Mesa, CA, U.S.A.), and amphotericin B 2.5 μg/mL (Sigma-Aldrich). All the cell cultures were maintained at 37˚C in a humidified atmosphere of 5% CO2. 3-(4,5-Dimethylthiazol-2-y1)- 2,5-diphenyl tetrazoliumbromide (MTT) was purchased from Sino-American Biotec.
2.2 Preparation of dendrimer modified SWCNT nanocomposites
As shown in Scheme 1, pristine SWCNTs were added to H2SO4/HNO3 (v/v=3:1). The mixture was placed in an ultrasonic bath for 60 min and then stirred for 24 h while being boiled under reflux. The mixture was then vacuum- filtered through a 0.22 μm Millipore polycarbonate membrane and subsequently washed with distilled water until the pH of the filtrate was ca. 7, yielding COOH- CNTs 0.2 g amine terminated PAMAM dendrimer were added to the solution. The solution was placed in an ultrasonic bath for 60 min and the mixture was stirred for 24 h at 50˚C. The mixture was subsequently filtered and washed three times with water. The product was dispersed in water to give a SWCNT-PAMAM dendrimer aqueous solution.
2.3 Characterization of dendrimer-modified SWCNTs by HR-TEM
The average particle size, size distribution and morpho- logy of SWCNTs and dendrimer coated SWCNTs were examined using a high resolution transmission electron microscope ((Hitachi H-700H) at a voltage of 80 kV. The aqueous dispersion of the particles was drop-cast onto a carbon coated copper grid and the grid was air dried at room temperature before viewing under the microscopy.
2.4 In vitro cell viability evaluation of dendrimer-modified SWCNTs by MTT
To determine cell viability, the MGC803 cells were planted at a density of 1×104 cells/well in 96 well plate at 37˚C in 5% CO2 atmosphere). After 24 h of culture, the medium in the wells was replaced with the fresh medium containing dendrimer coated SWCNTs in concentration range 0-2.0 mg/ml. After 24 h, 20 ml of MTT dye solution (5 mg/ml in phosphate buffer pH = 7.4) was added to each well. After 4 h of incubation at 37˚C, the medium was removed and formazan crystals were solubilised with 200 ml of dimethylsulphoxide (DMSO) and the solution was vigorously mixed to dissolve the reacted dye. After 15 min, the absorbance of each well was read on a microplate reader (DYNATECH MR7000 instruments) at 570 nm. The spectrophotometer was calibrated to zero absorbance, using culture medium without cells. The relative cell viability (%) related to control wells containing cell culture medium without nanoparticles was calculated by: Cell viability (%) = Atest/Acontrol × 100. Where Atest is the absorbance of the test sample and Acontrol is the absorbance of control sample.
2.5 Construction and identification of survivin siRNA vector
The pEGFP-U6-shRNA vector was presented as a gift by Professor Beate Brand-Saberi from Freiburg University, Germany. The vectors with anti-survivin SiRNA were constructed as follows: (a) the selected target sequence in the survivin gene (accession number: NM_001168) was from nucleotide 51 to 70. The target sequence was selected with the help of the Whitehead siRNA selection program (available at http://jura.wi.mit. edu/siRNAext; Bioinformatics and Research Computing at Whitehead Institute). A Blast search against the NCBI database revealed no significant homology of the targeted sequence to other genes (http://www.ncbi.nlm. nih.gov/BLAST). (b) as shown in Figure 1, as a survivin shRNA DNA template, one oligo sequence subsequently should include a sense sequence (5’-CACCG CATCT CTACA TTCAA GAATT CAAGA GATTC TTGAA TGTAG AGATG CTTTT TTG-3’), a loop sequence (5’ TTCAAGAGA 3’), and an antisense sequence (5’-GATCC AAAAA AGCAT CTCTA CATTC AAGAA TCTCT TGAAT TCTTG AATGT AGAGA TGC -3’). For ligation to the vectors to occur, two enzyme restriction sequences for BamHI (at the 5’ end) and Hind III (at the 3’end) must locate at both ends. For later, convenient analysis of the DNA plasmid constructs, an EcoR I restriction sequence should be added before the Hind III sequence. (c) All oligonucleotides were synthesized by the Ambion Company and purified using polyacrylamide gel. They were dissolved in an annealing buffer (10 mM Tris-Cl (pH 8.0)/50 mM NaCl/1 mM EDTA) and annealed by heating to and stepwise cooling as follows: 95˚C, 4 min; 90˚C, 4 min; 85˚C, 4 min; 80˚C, 4 min; 75˚C, 4 min; 70˚C, 4 min; 65˚C, 4 min; 60˚C, 4 min; 55˚C, 4 min; 50˚C, 4 min; 45˚C, 4 min; 40˚C, 4 min; 35˚C, 4 min; 30˚C, 4 min; 25˚C, 4 min. The oligonucleotide-duplexes codings for survivin and scrambled shRNAs were ligated to BamH I- and Hind III-cleaved pEGFP-U6-shRNA vectors to generate a pEGFP-U6-shRNA-survivin vector respectively. After that, the insertion sequences were confirmed by sequencing, and the control vectors were constructed according to and the method described in reference [25].
2.6 Dendrimer-coated SWCNTs mixing with pEGFP-U6-shRNA-survivin vector
The pH of the solution was 7.4. A suspension of the dSWCNTs at a concentration of 0.025 mg/ml was mixed with 1 μM pEGFP-U6-shRNA-survivin vectors for 2 h at room temperature prior to characterization or cellular incubation for intercellular uptake. The pEGFP-U6- shRNA-survivin vectors were adsorbed nonspecifically onto the dendrimer-modified SWCNTs.
2.7 Agarose gel electrophoresis
0.7% agarose gel electrophoresis was used to assess the formation of the dGN-pEGFP-U6-shRNA-survivin vector complex. The nanocomplexes were added to the wells of the ethidium-bromide agarose gel, the resulting gel was processed at 60 V for 20 min and then imaged using a Chemi-Doc system (Bio-Rad, Hercules, CA).
2.8 Cell culture and observation by fluorescence microscopy and HR-TEM
Human gastric cancer MG803 cells were cultured at 37˚C in a humidified 5 % CO2 and 95% air atmosphere in DMEM containing 1×105 mU/mL of penicillin and 0.1 mg/mL of streptomycin supplemented with 10% (v/v) FCS. The MGC803 cells were collected, seeded into 12 well plates, and cultured for 24 h as the experiment cells. Next, 100 μL dGN-pEGFP-U6-shRNA-survivin vector complexes were seeded into 6 well plates. Then, these vector complexes and the dendrimer-modified SWCNTs composites were seeded into other 6 well plates as the control cells. Finally, all the cells were cultured at 37˚C for 24, 48 and 72 h. After culturing, these cells were observed by fluorescent microscopy. Then, the MGC803 cells were collected and embedded into paraffin and made into TEM specimens, which then were observed via HR- TEM.
2.9 Effects of dendrimer modified SWCNTs- pEGFP-U6-shRNA on MGC803 cells
The MGC803 cells were cultured in a DMEM medium supplemented with 10% FBS and 1% penicillin- streptomycin at 37˚C for 48h. The MGC803 cells were collected and added into 24 well plates at the concentration of 5000 cells/well then, they were allowed to culture for 24 h. Next, the 0.025 mg/mL dGN-pEGFP- U6-shRNA vector nanocomplexes were added into the 24 well plates; at the same time, the control experiments with the dCNT-pEGFP-U6-shRNA vector or dCNT were set up, and continued to culture for 24, 48 and 72 h. MTT (5 mg/mL) was prepared in PBS and 20 μL was added to each well; the cells were incubated for 4 h at 37˚C, the medium was removed, 200 μL dimethyl sulfoxide (DMSO) was added to each well, and the optical density (OD) of each well was determined to be 515 nm, according to the microplate reader. The cell viability was calculated by the following formula [17]:
Cell viability (%) = ODthe treated cells / ODthe non-treated cells.
2.10 Statistical analysis
All data in this paper are presented as means result ±S.D. Statistical differences were evaluated using the t-test and considered significant at P < 0.01. In addition, all data in this paper were obtained from three independent experiments with similar results.
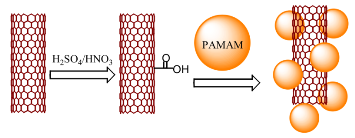
Scheme 1 Complexation between carboxyl SWCNTs and amine terminated PAMAM dendrimer.
3.1 Characterization of dendrimer coated SWCNTs
Size and morphology of SWCNTs and dendrimer coated SWCNTs were determined by TEM as shown in Figure 1. Figure 1a shows that the SWCNTs are ~10 nm in diameter with length over 1μm. The morphology of the SWCNTs after dendrimer coating was greatly changed as shown in Figure 1b. SWCNTs were shorten after HNO3 treatment from Figure 1b, the average length of CNTs is about 50 nm. Importantly, a thick layer of PAMAM dendrimer coated onto SWCNTs surface can be clearly observed, indicating the formation of dendrimer coated SWCNTs nanohybrid.
3.2 Effects of dendrimer-modified SWCNTs on MGC803 cells
To examine the effect of dendrimer-coated SWCNTs on MGC803 cell growth and viability, MGC803 cells were incubated with dendrimer-coated SWCNTs complex for 24 h, isolated by centrifugation and continued a 72 h incubation for MTT assay. Viable cells have the ability to reduce MTT from a yellow water-soluble dye to a dark blue insoluble formazan product. Formazan crystals were dissolved in DMSO and quantified by measuring the absorbance of the solution at 570 nm, and the resultant value is related to the number of living cells. Figure 2 demonstrated a dose-dependent reduction in MTT absorbance in cells treated with dendrimer-coated SWCNTs (concentration range 0-1.0 mg/ml) for 24 h. As shown in Figure 2 (b)~(e), Dendrimer coated SWCNTs showed no cytotoxic effects to cells and the cells remained more than 99% viable relative to control at concentration as high as1.0 mg/ml. No appreciable cell death was observed in the case of dendrimer coated SWCNTs. Theses results indicate that the PAMAM dendrimer coated SWCNTs exhibit little toxicity to MGC803 cells. The SWCNTs, however, was found to cause cell death when examined at 72 h after the 24 h incubation with MGC803 cells (Figure 2a). The degree of cell death was substantial as evidenced by the large amounts of cell debris observed. SWCNTs caused a significant reduction (96% of control) in cell viability even at 0.2 mg/mL concentration tested, and induced further reductions at higher concentrations, reaching a plateau around 0.5 mg/ ml, and that at the highest concentration tested (1.0 mg/ ml) it resulted in about 13% loss of cell viability.
3.3 TEM observation of cellular uptake of dendrimer modified SWCNTs
To investigate the cellular uptake of the dendrimer coated SWCNTs in vitro by TEM characterization. The TEM images showed that the dendrimer coated SWCNTs are internalized within the MGC803 cells after 24 h incubation (Figure 3). Several electron lucent voids containing SWCNTs can be seen in the cytoplasm of the MGC803 cells forming the vacuoles. After 24 h incubation with MGC803 cells, the SWCNTs are inter- nalized inside the cells, indicating PAMAM dendrimers can enhance the efficacy of SWCNTs entering into MGC803 cells.
3.4 Construction and Identification of pCMV- EGFP-U6-shRNA vector
The pCMV-EGFP-U6-shRNA vector was successfully constructed, as shown in Figure 4. UU indicates the deoxythydimidine dimmer as has a 3’-TT overhang. Figure 5 shows the electrophoretic shift assay result of pCMV-EGFP-U6-anti-survivin plasmid and the control pCMV-EGFP-U6-shRNA plasmid digested with EcoR I. Compared with the control empty vector, the constructed pCMV-EGFP-U6-anti-survivin-shRNA plasmid has a 156 bp fragment, which is matched with the inserted survivin fragment, therefore, the pCMV-EGFP-U6- shRNA-survivin plasmids (survivin-siRNA vector) were successfully constructed. We also finished the electrophoretic shift assay result of survivin-siRNA vector in the absence and presence of dSWCNTs, which showed that the amine-terminated dSWCNTs composites could bind with survivin-siRNA vector and formed stable dSWCNT-survivin-siRNA composites. Zeta potential analysis showed that, after G4.0 dendrimer modified SWCNTs, zeta potential reached + 30 mV at pH7.0, which is due to the increasing positive charges of –NH3+ on the surface of SWCNTs. The dSWCNTs with positive charge can bind with survivin- siRNA vector with negative charge via electrostatic attraction. G4.0 dSWCNTs absorbed the maximal amount of survivin-siRNA vectors. Positively charged survivin- siRNA-dSWCNTs would be easily attached to negatively charged cell membrane to improve the endocytosis. More positive charges on the surface of survivin-siRNA- dSWCNTs make higher gene delivery efficiency.
3.5 Effects of survivin-siRNA-dSWCNTs on MGC80 3 cells
As shown in Figure 6A, G4.0 dSWCNTs can inhibit the growth of MGC803 cells with the inhibition rate of 8%, showing that the dSWCNTs are very low toxic to MGC803 cancer cells. Under identical condition, the survivin-siRNA-dSWCNTs composites have the inhibition rate of 65%, the control empty vector- dSWCNTs composites only have the inhibition rate of 7%, there exists statistical difference between two groups (P<0.01), showing that inserted survivin fragment was very effective. As shown in Figure 6C, G4 dSWCNTs- survivin-siRNA composites inhibited the growth of MGC803 cells in dose-dependent means. As shown in Figure 6D, the MGC803 cells exhibited strong green color, showing the positive expression of survivin-siRNA vector in MGC803 cells, and also indirectly suggesting that dendrimer-modified SWCNTs did not affect the survivin-siRNA vector expression in MGC803 cells. In summary, our results show that dendrimer coated SWCNTs may be one kind of good siRNA delivery system, which can be used for intracellular siRNA imaging and therapy.
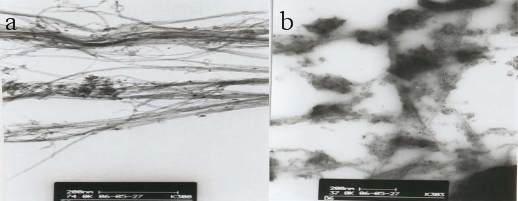
Fig. 1 TEM images of (a) SWCNTs and (b) G4.0 PAMAM dendrimer coated SWCNTs
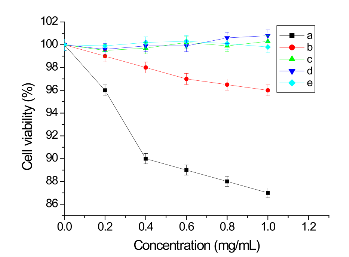
Fig. 2 Cytotoxicity profiles of SWCNTs and dendrimer coated SWCNTs, when incubated with MGC803 cells as determined by MTT assay. (a) SWCNTs, (b) G1.0 PAMAM dendrimer coated SWCNTs, (c) G2.0 PAMAM dendrimer coated SWCNTs, (d) G3.0 PAMAM dendrimer coated SWCNTs, (e) G4.0 PAMAM dendrimer coated SWCNTs.
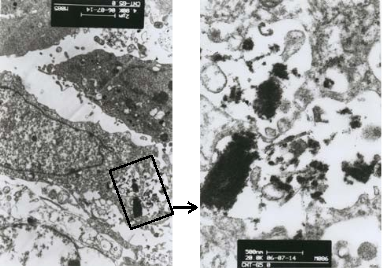
Fig. 3 TEM pictures of MGC803 cells incubated with G4.0 dendrimer coated SWCNTs showing SWCNTs internalization after 24 h incubation.
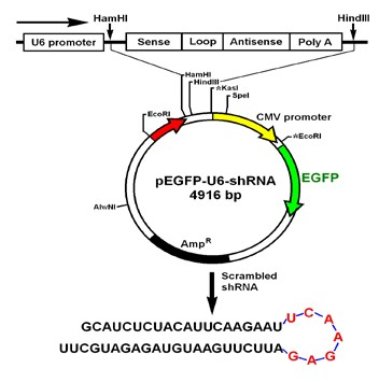
Fig. 4 Construction of pEGFP-U6-shRNA-survivin vector. Sequences of chemically synthesized anti-survivin, small hairpin RNAs (shRNAs) and their sense and antisense mRNA/cDNA target sequences.
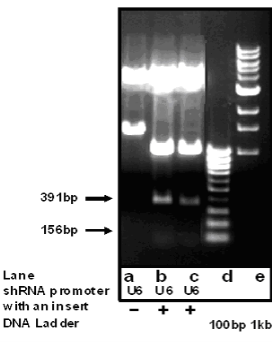
Fig. 5 Identification of pEGFP-U6-shRNA-anti-survivin siRNA vector cut with EcoR I by 0.7 % agarose gel electrophoresis. a pEGFP-U6- shRNA vector; b, c pEGFP-U6-shRNA-anti-survivin siRNA vector cut with EcoR I; d, e molecular markers.
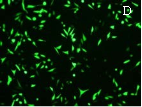
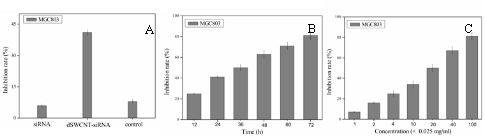
Fig. 6 Growth inhibition curves and fluorescent microscope observation of MGC803 cells. (A) dSWCNTs, siRNA-dSWCNTs and control at 24 h, (B) survivin siRNA-dSWCNTs composites at different time, (C) survivin-siRNA-dSWCNTs composites at different concentration dSWCNTs + 2μM siRNA vector, control: 0.025mg/mL dSWCNTs + 2μM dSWCNTs-pCMV-EGFP-U6-shRNA vectors .(D) Fluorescent microscope observation of MGC803 cells incubated with dSWCNTs-pCMV-EGFP-U6-shRNA vectors.( × 20).
In conclusion, dendrimer coated SWCNTs were successfully prepared, the colloidal solution of dendrimer coated SWCNTs showed high water-soluble, stability. The dendrimer coated SWCNTs were low cytotoxicity, which can bind with survivin siRNA vector via electrostatic interaction, resultant survivn siRNA SWCNTs can enter into MGC 803 cells high efficiently, the survivin siRNA vector can be released out from the nanocomposites of survivin siRNA vector-dSWCNTs, and inhibited the growth of tumor cells, the EGFP exhibited strong expression in MGC803 cells, the dendrimer modified SWCNTs may be one kind of delivery system for siRNA, and own great potential in applications such as gene or siRNA delivery, and intracellular imaging.
This work is supported by the National Key Basic Research Program (973 Project) (No. 2011CB933100), National Natural Scientific Fund (No.81225010, 31100717), 863 project of China (2012AA022703),
Shanghai Science and Technology Fund (No.13NM1401500), Shanghai Jiao Tong University Innovation Fund for Postgraduates (No. AE340011).
Copyright:(c) 2013 C. Wang et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credied.