Review
Extracellular Synthesis of Silver Nanoparticles by Endophytic Bordetella SP. Isolated from Piper Nigrum and Its Antibacterial Activity Analysis
Roshmi Thomas1*, B Jasim 1*, Jyothis Mathew 1, EK Radhakrishnan 1*
1 School of Biosciences, Mahatma Gandhi University, PD Hills (PO), Kottayam, Kerala, India – 686 560.
* Corresponding authors. E-mail: radhakrishnanek@mgu.ac.in Tel/fax.: + 91 9847901149
Citation: R. Thomas, et al. Extracellular synthesis of sliver nanoparticles by endophytic Bordetella sp. isolated from Piper nigrum and its antibacterial activity analysis . Nano Biomed. Eng. 2012, 4(4), 183-187.
DOI: 10.5101/nbe.v4i14.p183-187.
Abstract
In the current study, various endophytic bacterial isolates from Piper nigrum were explored for its nanoparticle synthesizing property. Very interestingly, one of the isolate which is a strain of Bordetella sp. was found to have the ability to form silver nanoparticles at room temperature within 24 h by extracellular method. This was confirmed by the presence of a peak at 460 nm in the UV- Vis spectrum and such a result is the indication of surface plasmon resonance of silver nanoparticles. Further characterization of nanoparticles was carried out by Scanning electron microscopy (SEM) analysis. This confirmed the presence of silver nanoparticles with size less than 100 nm. The antibacterial potential of silver nanoparticles synthesized was also tested against pathogens like Salmonella paratyphi, Vibrio cholerae and Staphylococcus aureus. So the current study is the demonstration of an efficient synthesis of silver nanoparticles by Bordetella sp. and their potential as nanomedicine to manage pathogenic bacteria.
Keywords: Antibacterial activity, Biosynthesis, Endophytic, Extracellular, Silver nanoparticles
Size dependent properties exhibited by the nano particles make them to have attractive medical and technological applications. Nanoparticles differ signi ficantly from the bulk materials in its physical, chemical and biological properties due to their large fraction of surface atoms, large surface energy and spatial confinement. In addition to its advantages as nontoxic, and environmentally benign synthetic procedures, biological methods of nanoparticle synthesis provides particles with good control over the size distribution [1,2]. Various groups of microorganisms like bacteria, actinomycetes, yeast and fungi are exploited for their potential to form metal nanoparticles. These nanoparticles especially silver nanoparticles (AgNPs) are well known for their potent biological properties [3-5]. So it is of great importance to explore novel microbial sources for its potential to synthesize AgNPs. Although endophytic microbes are greatly investigated for their bioactive principles, their use as mediators for AgNPs is very limited. Biosynthesis of AgNPs by Pseudomonas stutzeri AG 259 was the first report on bacterial synthesis of AgNPs [6,7]. Metal nanoparticles synthesized by microorganisms as a result of reduction of metal to its element form can get accumulated intracellularly or extracellularly [8-12]. The cell wall of microorganisms plays a major role in intracellular nanoparticle synthesis. After the interaction, enzymes present in the cell wall bioreduce the metal ions to nanoparticles[13]. But extracellular method for the synthesis of metal nanoparticles by particular microbial source involve metal reduction by cell wall reductive enzymes or soluble secreted enzymes especially nitrate reductase [10,14]. Out of these two possible methods, extracellular production of metal nanoparticles is of greater commercial applications due to the advantages in its purification [1]. Various groups of bacteria are reported for their ability to form AgNPs extracellularly [15,16]. Members of Enterobacteriaceae like K. pneumoniae, E. coli and E. cloacae are known to form AgNPs with a size range of 28.2 nm to 122 nm by extracellular reduction of Ag (+) to Ag(0) [17]. Bacillus megaterium was also known to have the ability to synthesize AgNPs by extracellular methods within few minutes [18]. Since the extracellular microbial synthesis of AgNPs is much attractive, studies on these properties from endophytic bacterial isolate is very important. As the silver in nanometric scale has received significant use as antimicrobials, screening its effect on various pathogens can have many applications. Effect of AgNPs on viability of Bacillus subtilis, Enterococcus faecalis, Escherichia coli, Salmonella typhimurium and Candida albicans indicate its promising uses [19]. In addition to this, extracellularly synthesized AgNPs by Bacillis megaterium were found effective against multi drug resistant organisms including S. pneumoniae and S. typhi [18]. The activity of AgNPs against methicillinresistant Staphylococcus aureus, methicillin-resistant Staphylococcus epidermidis and Streptococcus pyogenes are also reported [20]. All these support the importance of screening diverse microorganisms for their ability to synthesize AgNPs which can have much antimicrobial potential. In our preliminary search for endophytic bacteria from Piper nigrum, we have identified seven isolates based on 16SrDNA sequence analysis (unpublished data). In the current study, all the identified endophytic bacterial isolates were screened for its ability to form AgNPs. Very interestingly, one of the isolate which belong to Bordetella sp. was found to form AgNPs by the reduction of metal ions. The formation of AgNPs was monitored by visual inspection and also by measuring the UV-Visible spectra. This was further confirmed by scanning electron microscopy analysis. The biosynthesized AgNPs were further used to study its antimicrobial effect on Salmonella paratyphi, Vibrio cholerae and Staphylococcus aureus. The result of which confirms the importance of screening endophytic bacterial communities for it nanoparticle synthesizing properties and the application of synthesized AgNPs.
2. Materials and Methods
The endophytic bacterial isolates from Piper nigrum obtained in our previous study were used for the synthesis of AgNPs. Out of the seven endophytic bacterial isolates (PnB1-PnB7) used, PnB4 was found to have the ability to form AgNPs and this isolate was confirmed as strain coming under Bordetella sp. by by 16S rDNA sequencing analysis.
2.1. Synthesis of silver nanoparticles
The selected isolates were inoculated separately into 100 mL of nutrient broth and were incubated for 24 h at 200 rpm in a rotating shaker at room temperature. The biomass and supernatant separated from the culture were used for the intracellular and extracellular synthesis of AgNPs respectively. For this, the biomass and supernatant were collected by centrifugation for 10 min at 10000 rpm. For supernatant assisted production of AgNPs, one ml of bacterial supernatant was mixed with 99 mL of filter sterilized 1mM AgNO3. But for biomass assisted synthesis, bacterial wet biomasses were mixed with aqueous solution of 1 mM AgNO3 in a range of 100 mL of 1 mM AgNO3 for 2 g of biomass. All the mixtures were incubated, for 72 h at room temperature in light and dark conditions, on a rotating shaker at 200 rpm. As the experimental control, heat killed samples like biomass and supernatant with silver nitrate and silver nitrate solution alone were also maintained. The formation of AgNPs was visually observed by checking the colour change. This was further monitored by analyzing the optical characteristics of synthesized of AgNPs using UV-visible spectrophotometer (Hitachi U5100) at a range of 200-800 nm with control as reference.
2.2. Characterization of AgNPs
AgNPs produced by the selected isolate were air-dried and analyzed using scanning electron microscopy. SEM analysis of the synthesized nanoparticles was conducted by mounting the dried samples on specimen stubs with double adhesive tape and coated with platinum in a sputter coater. This was then observed under JEOL 6390 SEM JSM at 10 KV.
2.3. Antibacterial activity analysis of AgNPs
The antibacterial potential of the synthesized AgNPs was analysed by agar well diffusion assay method using Mullor Hinton Agar (MHA) plates [18,19]. The test organisms used for the study were Salmonella paratyphi, Vibrio cholerae, and Staphylococcus aureus. They were cultured in Muller Hinton broth for 24 h at 37oC and from this a confluent lawn of bacterial culture was made on MHA agar plates by swabbing. Then wells were made on these plates using a gel puncture and approximately 40 µL of the synthesized AgNPs solution was added into each well. As a control 40 µL of 1 mM AgNO3 solution was added in to the well on MHA agar plate. All the plates were incubated at 37oC for 24 h and the diameter of zone of inhibition was measured.
3. Results and Discussion
Although endophytic bacteria were greatly studied for its bioactive compounds and also for its use as biocontrol agent, its studies on nanoparticle synthesis is very limited. Among the seven bacterial isolates (PnB1 to PnB7) used for the study, culture supernatant of Bordetella sp. (PnB4) was found to have the potential to form AgNPs. This was indicated by the colour change of the supernatant when incubated with 1 mM AgNO3 (Fig. 1). This colour change which is due to the excitation of surface plasmon resonance of AgNPs is a well known proof of AgNPs formation [18,21]. The absence of significant colour change for the biomass assisted reactions either in the presence or absence of light and also considering the advantages with the extracellularly generated nanoparticles, further studies were conducted based on the AgNPs synthesized by the culture supernatant. Also this confirmed that the reduction of the Ag+occurs only under visible-light irradiation. The impact of visible light irradiation on the biosynthesis of AgNPs is already reported [22,23]. The absence of colour change in the heat killed control of the isolates used for the study under the same experimental condition confirmed the colour change as indication of AgNPs formation. The similar observations were previously reported for the AgNPs formation by cultures of Bacillus sp. where the colour change was confirmed as occurred due to reduction of aqueous silver ions to AgNPs [10,24]. The production of AgNPs was further monitored by UV-Vis spectral analysis, as part of its preliminary characterization [25].The UV-visible spectra of AgNPs formed by Bordetella sp. (PnB4) after 24 hours showed a strong peak at 460 nm (Fig. 2). Such an absorption spectrum of AgNPs is due to its surface plasmon excitation as a result of the collective excitation of conduction electron in metal. Peak formation at this range is previously known with the AgNPs synthesized by Neurospora crassa and Bacillus licheniformis [26,10]. The lower wavelength absorbance in UV-Vis spectrum is due to the protein involved in the synthesis and stabilization of AgNPs [24]. For further confrmation of AgNPs, the samples were resuspended in deionized water and were used for SEM analysis. The SEM micrograph of purifed nanoparticles showed the presence of more or less spherical AgNPs with size ranging from 63-90 nm (Fig. 3). The size ranges of AgNPs produced by Bordetella sp. fall closer to the size of silver reported for other bacteria [8,27]. For screening the antibacterial activity of AgNPs synthesized by the endophytic Bordetella sp., both gram positive (Staphylococcus aureus) and gram negative (Salmonella paratyphi and Vibrio cholerae) bacteria were used as test organisms. The biosynthesized AgNPs at the volume of 40 µL/well showed antibacterial act-ivity against all tested bacterial strains (Fig.4). For Salmonella paratyphi it showed zone of inhibition with a diameter of 14 mm, for Vibrio cholerae it was 13 mm and for the Staphylococcus aureus it was 14 mm (Table 1). Comparative analysis of zone of inhibition by the synthesized AgNPs with the control, the antimicrobial activity of synthesized silver nanoparticles can be confirmed. AgNPs were previously known to have antibacterial activity against Staphylococcus aureus [18] and Vibrio cholerae [28] and this further confirm the observed results. The small size and large surface area to volume ratio of the AgNPs may favor its bactericidal activity [29].
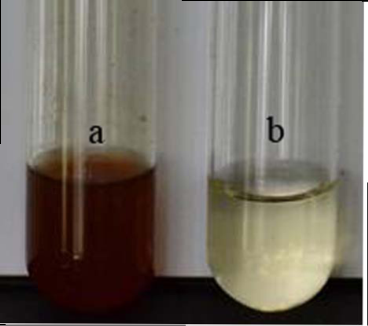
Fig. 1 Visual observation of the biosynthesis of AgNPs by Bordetella sp. selected for the study along with control after 24 h of incubation. (a): bacterial supernatant with 1mM AgNO3 solution (color change from pale yellow to brown), (b): heat killed supernatant with AgNO3 solution (no color change).
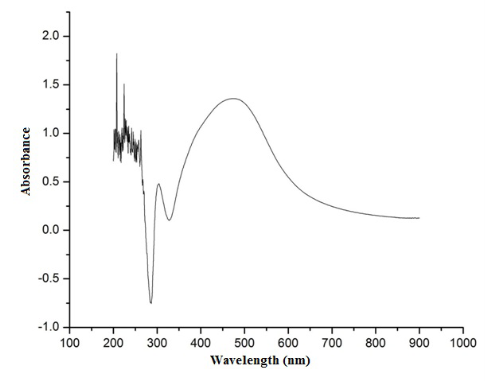
Fig. 2 The UV-Vis spectrum of AgNPs synthesized by the supernatant of Bordetella sp. The absorption spectrum of AgNPs exhibited a strong broad peak at 460 nm.
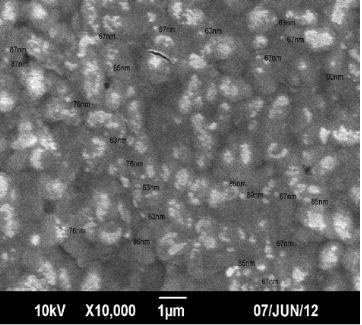
Fig. 3 SEM image of AgNPs synthesized by Bordetella sp. with 1 mM AgNO3 solution
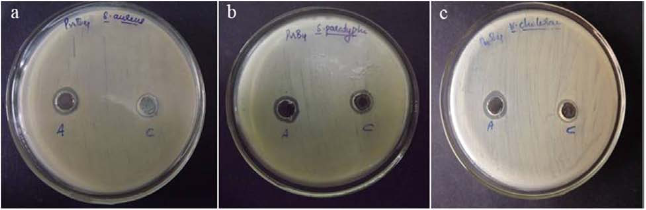
Fig. 4 Antimicrobial activity of AgNPs synthesized by Bordetella sp. against (a) Staphylococcus aureus (b) Salmonella paratyphi (c) Vibrio cholerae. Each plate shows (A) AgNPs synthesized by Bordetella sp. (C) 1 mM AgNO3 control
Table 1 Diameter of zone of inhibition against pathogenic gram positive and gram negative bacteria by synthesized AgNPs

4. Conclusions
The variation in physico-chemical properties of the nanoparticles synthesized through diverse microbial sources can affect its applications. This indicates the importance of exploration of novel untapped bacterial sources for the identification of potential strains with nanoparticles synthesizing property. Endophytes by occupying the unique habitat, is greatly unexplored in terms of its nanoparticle synthesizing properties. Among the seven endophytic bacterial isolates screened for its ability to synthesize AgNPs, one of them which belong to the genera Bordetella was found to have promising applications as indicated by the formation of AgNPs. The nanoparticles formed by the isolate were also found to have remarkable inhibition against pathogens.
Acknowledgements
The authors gratefully acknowledge School of Chemical Sciences, Mahatma Gandhi University, Kottayam, Kerala, India for the help and support for the SEM analysis of samples and also to DBT-RGYI and DST-PURSE programme for the instrumentation facility.
References
1. Narayanan K.B., Sakthivel N., Biological synthesis of metal nano-particles by microbes. Adv. Colloid Inter. Sci. 2010; 156:1-13.
2. Pugazhenthiran N., Anandan S., Kathiravan G., Prakash N.K.U., Crawford S., Ashokkumar M., Microbial synthesis of silver nano-particles by Bacillus sp. J. Nanopart Res. 2009;11: 1811-1815.
3. Elechiguerra J.L., Burt J.L., Morones J.R., Camacho-Bragado A., Gao X., Lara H.H., Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 2005; 3: 6-16.
4. Wei H., Chen C., Han B., Wang E., Enzyme colorimetric assay using unmodifed silver nanoparticles. Anal.Chem. 2008; 80: 7051-7055.
5. Christopher P., Xin H., Linic S., Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nat. Chem. 2011; 3: 467-472.
6. Haefeli C., Franklin C., Hardy K., Plasmid-determined silver resistance in Pseudomonas stutzeri isolated from silver mine. J. Bacteriol. 1984; 158: 389-392.
7. Klaus T., Joerger R., Olsson E., Granqvist C.G., Silver-based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. USA. 1999; 96: 13611-13614.
8. Ahmad R., Minaeian S., Shahverdi H.R., Jamalifar H., Nohi A., Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: A novel biological approach. Proc. biochem. 2007; 42: 919-923.
9. Jain N., Bhargava A., Majumdar S.,Tarafdar J.C., Panwar J., Extracellular biosynthesis and characterization of silver nano-particles using Aspergillus favus NJP08: a mechanism perspective. Nanoscale. 2011; 3: 635-641.
10. Kalimuthu K., Babu R.S., Venkataraman D., Bilal M., Gurunathan S., Biosynthesis of silver nanocrystals by Bacillus licheniformis.Colloids Surf. B.2008; 65:150-153.
11. Saifuddin N., Wong C.W., NurYasumira A.A., Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. J. Chem. 2009; 6: 61-70.
12. Thomas R., Viswan A., Mathew J., Radhakrishnan E.K., Evaluation of antibacterial activity of silver nanoparticles synthesized by a novel strain of marine Pseudomonas sp. Nano Biomed. Eng. 2012; 4: 139-143.
13. Mukherjee P., Ahmad A., Mandal D., Senapati S., Sainkar S.R., Khan M.I., Ramani R., Parischa R., Ajaykumar P.V., Alam M., Sastry M., Kumar R. Bioreduction of AuCl4- ions by the fungus Verticillium sp.and surface trapping of the gold nanoparticles formed. Angrew. Chem. Int. Ed. 2001; 40:114-117.
14. Anil Kumar S., Abyaneh M.K., Gosavi Sulabha S., Ahmad A., Khan M.I., Nitrate reductase mediated synthesis of silver nano-particles from AgNO3. Biotechnol. Lett.2007; 29:439-445.
15. Shivaji S., Madhu S., Singh S., Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Process biochem. 2011; 49: 830-837.
16. Nanda A., Saravanan M., Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomedicine. 2009; 5: 452-456.
17. Shahverdi A.R., Minaeian S., Shahverdi H.R., Jamalifar H., Nohi A.A., Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteriaceae: a novel biological approach. Process Biochem. 2007; 42: 919-923.
18. Saravanan M., Venu A.K., Barik S.K., Rapid biosynthesis of silver nanoparticles from Bacillus megaterium (NCIM 2326) and their antibacterial activity on multi drug resistant clinical pathogens. Colloids Surf. B. 2011; 88: 325-331.
19. Sadhasivam S., Shanmugam P., Yun K., Biosynthesis of silver nanoparticles by Streptomyces hygrocopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloids Surf. B. 2010, 81: 358-362.
20. Shameli K., Ahmad M.B., Zargar M., Yunus W.M.Z., Ibrahim N.A., Shabanzadeh P., Moghaddam M.G., Synthesis and characterization of silver/montmorillonite/chitosan bionanocomposites by chemical reduction method and their antibacterial activity. Int. J. Nanomedicine.2011; 6: 271-284.
21. Ahmad A., Mukherjee P., Senapati S., Mandal D., Khan M.I., Kumar R., Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B. 2003; 28: 313-318.
22. Mokhtari N., Daneshpajouh S., Seyedbagheri S., Atashdehghan R., Abdi K, Sarkar S., Minaian S., Shaverdi H.R., Shaverdi A.R, Biological synthesis of very small silver nanoparticles by culture supernatant of Klebsiella pneumonia: The effects of visible-light irradiation and the liquid mixing process. Mater. Res.Bull. 2009; 44: 1415-1421.
23. Wei X., Luo M., Li W., Yang L., Liang X., Xu L., Kong P., Liu H.,Synthesis of silver nanoparticles by solar irradiation of cell-free Bacillus amyloliquefaciens extracts and AgNO3. Biores. technol. 2012; 103: 273-278.
24. Babu G., Gunasekaran P., Production and structural characterization of crystalline silver nanoparticles from Bacillus cereus isolate. Colloids Surf. B. 2009; 74: 191-195.
25. Sastry M., Patil V., Sainkar S.R., Electrostatically controlled diffusion of carboxylic acid derivatized silver colloidal particles in thermally evaporated fatty amine flms. J. Phys. Chem. B 1998; 102: 1404-1410
26. Longoria E.C., Nestor A.R.V., Borja M.A., Biosynthesis of silver, gold and bimetallic nanoparticles using the filamentous fungus Neurospora crassa. Colloids Surf. B. 2011; 83: 42-48.
27. Gurunathan S., Kalishwaralal K., Vaidyanathan R., Deepak V., Pandian S.R.K., Muniyandi J.H., Hariharan N., Eom S.H., Biosynthesis, purifcation and characterization of silver nanoparticles using Escherichia coli. Colloids Surf. B. 2009; 74: 328-335.
28. Renugadevi K., Aswini R., Microwave irradiation assisted synthesis of silver nanoparticle using Azadirachta indica leaf extract as a reducing agent and in vitro evaluation of its antibacterial and anticancer activity. Int.J. Nanomat. Bio. 2012; 2: 5-10.
29. Baker C., Pradhan A., Pakstis L., Pochan D.J., Shah S.I., Synthesis and antibacterial properties of silver nanoparticles. J Nanosci. Nanotechnol. 2005; 5: 244-249.
Copyright:(c) 2012 R. Thomas, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.