Article
Free Radical Scavenging and Antioxidant Activity of Silver Nanoparticles Synthesized from Flower Extract of Rhododendron dauricum
Amit Kumar Mittal, Abhishek Kaler and Uttam Chand Banerjee *
Department of Pharmaceutical Technology (Biotechnology), National Institute of Pharmaceutical Education and Research, Sector-67, SAS Nagar – 160062 Punjab, India.
* Corresponding author. Email: ucbanerjee@niper.ac.in Tel: +91-172-2214682-87, Fax: +91-172-2214692.
Citation: Amit Kumar Mittal, et al. Free Radical Scavenging and Antioxidant Activity of Silver Nanoparticles Synthesized from Flower Extract of Rhododendron dauricum . Nano Biomed. Eng. 2012, 4(3), 118-124.
DOI: 10.5101/nbe.v4i3.p118-124.
Abstract
A simple and efficient eco-friendly approach for the biosynthesis of stable, monodisperse silver nanoparticles (AgNPs) using Rhododendron dauricum flower extract is described. Different reaction parameters (concentration of plant extract, substrate concentration, pH, temperature and reaction time) were optimized to synthesize AgNPs with controlled properties. AgNPs were characterized in terms of synthesis, size distribution (PDI of 0.25), capping functionalities (phenolic compounds) and microscopic evaluation by UV-Visible spectroscopy, dynamic light scattering, Fourier Transform Infrared (FTIR) spectroscopy and Transmission Electron Microscope (TEM). The specific characteristics and loss of organic content (1.81 mg) of the synthesized nanoparticles was measured by Differential Scanning Calorimetry (DSC) and Thermo Gravimetric Analysis (TGA). The results showed the simplistic and feasible approach for obtaining stable aqueous monodispersive AgNPs. Further, the antioxidant activity of AgNPs imparted by plant phenolic components was evaluated using DPPH assay and found to be comparable to standard TROLOX.
Keywords: Green synthesis, Silver nanoparticles, Plant extracts, Antioxidants assay
1. Introduction
Silver nanoparticles (AgNPs) have a wide range of applications in various fields from electronics to biology, pharmaceuticals to medical diagnosis and therapeutics to biosensor development [1,2]. To maximize the use of nanosized metal nanoparticles (MNPs), a large spectrum of research has been focused to control the size and shape that reflects in their chemical and optical properties [3]. Various physical and chemical methods were extensively used to synthesize monodispersed AgNPs [4]. Silver NPs synthesized by the reduction of aqueous Ag+ ions using plant extract has been reported in literature [5,6]. This green chemistry approach towards the synthesis of AgNPs has many advantages such as, less reaction time (few minutes) compared to hours taken by microbial counterpart [7,8]. Cost effectiveness, ease of scale up, economic viability and most importantly eco-friendly nature [9]. Certain reports highlighting role of plant constituents in formation and stabilization of AgNPs have been published [10-13]. In the present study, silver nanoparticles have been synthesized using flower extract of Rhododendron dauricum. Water was used as the environment benign reaction medium and plant metabolites as reducing and capping agent, making the process greener one. Interaction between metal nanoparticles and capping agents is not much documented and properties that nanoparticles may acquire as a result of these interactions are even less reported. Keeping in view the antioxidant constituents present in R. dauricum flower part and their possible interaction with metallic surface, we investigated the antioxidant potential of silver nanoparticles capped by these components.
2. Materials and Methods
2.1 Reagents
Silver nitrate used for biosynthesis of AgNPs was purchased from Sigma-Aldrich (Steinheim, Germany). Fresh Rhododendron dauricum flowers were purchased from the flower market, Sector-26, Chandigarh, India. Incubator Shaker (Innova 4230) and UV-Vis Spectro- photometer (U-3010, HITACHI) were used to characterize AgNPs formation. DPPH (1,1-diphenyl-2-picrylhydrazyl) and TROLOX (2,2′-azino-bis (3-ethylbenzthiazoline-6-
sulphonic acid)) were purchased from Sigma-Aldrich, USA.
2.2 Preparation of the flower extract
Flower parts of Rhododendron dauricum were thorou- ghly washed and dried completely in the shade. Dried parts were cut into small pieces and powdered in a grinder. Fifty gram of powder was suspended in 500 mL deionized water and extracted at 50 oC for 120 min. The extract was filtered and stored at 4 oC for NPs synthesis.
2.3 Green synthesis and characterization of AgNPs
The aqueous flower extract of R. dauricum was used in the bioreduction of silver ions. In a typical experiment, AgNO3 was added to aqueous solution of plant extract. The reaction was allowed to proceed at 35 oC and 200 rpm in the dark environment. Two controls were used (one without flower extract i.e., only silver nitrate in deionised water and other without silver nitrate i.e. only plant extract in deionised water). The reaction mixture was periodically observed for the change in colour and analyzed by UV-Vis spectrophotometer in the range of 300-800 nm. The size and potential of nanoparticles were estimated by Zeta-Sizer. Nanoparticles were harvested using centrifugation (7000×g, 15 min) and washed thrice using deionised water to remove plant extract material. Pellet of nanoparticles was resuspended in deionised water and freeze dried using Lyophilizer. The functional groups capping of the synthesized NPs was confirmed by FTIR Spectroscopy. The size and shape of AgNPs was determined using High Resolution Transmission Electron Microscope. The stability and degradation of plant extract capped NPs was assessed using Differential Scanning Calorimetry and Thermo Gravimetric Analysis.
2.4 Optimization of various parameters to increase the nanoparticles synthesis
2.4.1 Concentration of plant extract
Ratio of metal ions to reducing agent affects the rate of synthesis as well as the size and shape of nanoparticles. To synthesize NPs from flower extract of R. dauricum different concentrations of extract were prepared from stock solution. Concentrated extracts (1 to 10 mL) were diluted to 100 mL with deionised water to prepare reducing solution of different concentrations. Silver salt similarly reaction mixture having initial pH in the range of 3 to 13 was incubated at 35 oC under shaking condition (200 rpm) keeping other parameters constant.
2.4.2 Silver ion concentration
To optimize the initial silver ion concentration, the flasks were incubated (35 oC, 2000 rpm) containing different concentrations of AgNO3 (0.5-5 mM) with fixed concentration of plant extracts (0.02 dilution) in dark condition.
2.4.3 Temperature and pH
Reaction flasks containing plant extract (0.02 dilution) incubated and 4 mM AgNO3 were incubated at temperatures ranging from 25-65 oC under shaking condition (2000 rpm).
2.4.4 Reaction time
To optimize reaction time in terms of yield and properties of nanoparticles, samples were collected at various time intervals (6, 12, 24, 36, 48 and 72 h.) from the reaction mixture and subjected to further characterization.
2.5 Free radical (anti-oxidant) scavenging activity: DPPH assay
Free radical (anti-oxidant) scavenging activity of the AgNPs was determined by the DPPH assay [14]. Ethanolic solution of DPPH was prepared to a final concentration of 200 μM and served as negative control. Similarly different concentrations of AgNO3 (25-100 μg mL-1) in water were prepared. Reaction mixture was prepared by adding 100 μL silver nanoparticle having a range of concentration into 100 μL DPPH solution. The reaction mixture was incubated for half an hour at room temperature under shaking condition and the absorbance was measured at 517 nm in 96 well plate. The change in absorbance with respect to the control (containing DPPH only) is calculated as percentage scavenging activity. TROLOX solution was used as positive control. The assay was performed in duplicate.
3. Results and Discussion
3.1 Visual inspection and UV-Vis spectroscopy analysis
Due to Surface Plasmon Resonance, AgNPs show yellowish brown to dark brown colour, depending upon their size and shape [15]. Preliminary identification of nanoparticle formation was carried out by observing the colour change of the reaction solution. Colloidal solution of AgNPs was subjected to UV-Vis spectral analysis where sharp bands of silver colloids were observed at 400-450 nm (Fig. 1a) as previously reported by other workers [5,16]. Two control reactions were kept as shown in Fig. 1b i.e. aqueous solution of AgNO3 which is without any colour and plant extract solution that exhibit pale yellow colour. Reaction mixture containing AgNO3 and plant extract shows brown colouration indicating formation of AgNPs in the reaction mixture. Parallel control experiments didn’t show any absorption at 420 nm. The stability of the AgNPs was studied for a period of three month. UV-Vis spectral analysis of lyophilized silver nanoparticles was carried out to check its stability after dispersion in water. The results showed similar monodispersity and chemical stability of the nanoparticles which are well dispersed in solution without any aggregation.
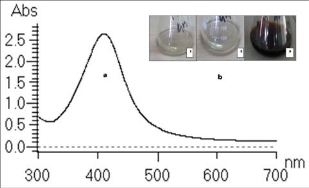
Fig. 1 UV-Vis spectrum of (a) AgNPs produced by reaction of plant extract with silver nitrate (1 mM) in 24 h. (b) aqueous solution ofAgNO3 (control 1), plant extract solution without silver nitrate (control 2) and reaction mixture (3) incubated at 35 oC, 200 rpm
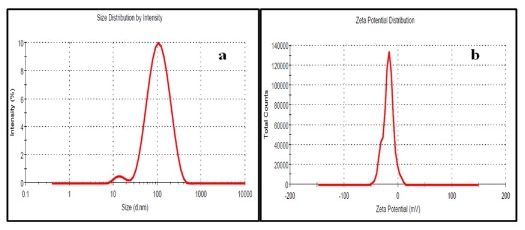
Fig. 2 (a) Particle size distribution and (b) zeta potential of silver nanoparticles synthesized by flower extract of Rhododendron dauricum
3.2 Zeta sizer
The average hydrodynamic size (Fig. 2a) and zeta potential (Fig. 2b) of AgNPs in aqueous solution was found to be 89 nm and -18 mV, respectively. PDI of 0.25 indicates good monodispersity and zeta potential indicates capping of NPs by negatively charged groups (Fig. 2b).
3.3 TEM analysis
A representative TEM image of the prepared AgNPs is shown in the Fig. 3. TEM image shows that the synthesized NPs lie in the size range of 25-40 nm and capped by plant constituents that prevented their aggregation. Silver nanoparticles of similar size were prepared by Cinnamomum camphora leaf extract [17]. Inherent capping offers additional advantage of the stability in the green chemical synthesis (Fig. 3) [18]. This stability attributes of the plant constituents are in consensus with the reports by Ahmad et. al. [19].

Fig. 3 TEM image of silver nanoparticles synthesized by flower extract of R. dauricum
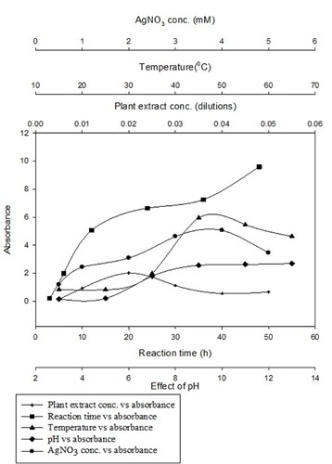
Fig. 4 Effect of various reaction parameters on AgNPs synthesis by water extract of R. dauricum flower
3.4 Optimization of nanoparticle synthesis
3.4.1 Concentration of plant extract
Results of optimization studies are shown in Fig. 4. It is reported [9] in literature that optimum extract to metal salt ratio is required for the synthesis of symmetrical NPs. Diluted flower extract containing 2 mL extract in 100 mL water i.e. 0.02 dilution was able to produce the maximum concentration of AgNPs as observed by higher absorbance at 420 nm. Peak observed at 0.02 dilution of plant extract was sharp as compared to other peaks. This indicates the formation of relatively monodispersed particles when 0.02 dilution of plant extract was used.
3.4.2 Silver ion concentration
Effect of initial AgNO3 concentration was studied by varying the concentration of AgNO3 from 0.5- 5 mM using 0.02 dilution of plant extract (Fig. 4). Increase in yield of AgNPs was observed when metal salt concentration was increased from 0.5-4 mM. Beyond this there was again fall in absorbance; hence 4 mM concentration of AgNO3 was selected for further experiments. Similar effect of varying concentration of plant extract and silver salt on yield, size and dispersity of silver nanoparticles was reported by Dwivedi et. al. [20].
3.4.3 Temperature and pH
Both reaction temperature and pH play significant role to control the nucleation process of nanoparticle formation. Increase in absorbance of reaction mixture with the increase in incubation temperature evidently depicts the higher synthesis of AgNPs at elevated temperatures [21]. Absorbance increased with increase in the temperature from 25 to 45 oC and thereafter decreased at higher temperatures (Fig. 4). Moreover, nanoparticles synthesized at higher temperature exhibit surface Plasmon resonance at narrow absorption range indicating monodispersity. Increase in temperature increased the rate of formation of silver nanoparticles from silver ions, retarding the secondary reduction process. Similar results have been reported by Song et.al. [22] and Kaviya et.al. [23]. Effect of reaction pH on the ability of reductant to synthesise and stabilize metal nanoparticles was reported by Selvakannan et.al. [24]. Effect of pH on the synthesis of AgNPs by R. dauricum was tested over a wider pH range (pH 3-13) (Fig. 4). At acidic pH, larger size NPs were formed, whereas, at alkaline pH, smaller size NP formation was observed. Sathishkumar et.al. [25] reported similar effects of pH on the size and shape of silver nanoparticles. Nanoparticle aggregation seems to outdo the nucleation process in acidic conditions. At alkaline pH, however, the large numbers of nuclei formation, instead of aggregation, led to the synthesis of more of NPs with smaller diameter. Blue shift in absorption pattern confirmed formation of relatively smaller nanoparticles. This result indicates the very important role played by reaction pH in controlling the shape and size of the AgNPs synthesis.
3.4.4 Reaction time
Absorbance of silver colloidal solution increased with span of time and maximum absorption was observed after 12 h of reaction (Fig. 4). Reaction time of more than 10 h is due to less redox potential of silver ions. These findings are in agreement with observations made by Singh et. al. [26]. Aggregation of NPs after 36 h of reaction was observed, a sign of instability.
3.5 FTIR spectroscopy analysis
It has been reported [27] in literature that plant constituents involved in the reduction and capping of nanoparticle can be identified by FTIR technique. FTIR measurements were taken to identify the possible biomolecules responsible for capping and efficient stabilization of the AgNPs synthesized by R. dauricum flowers. Fig. 5 shows the FTIR spectra of flower extract of R. dauricum and synthesized AgNPs overlay. The AgNPs sample shows peaks at 3418, 2920, 1604, 1432, 1384, 1053 and 569 cm−1 (Fig. 5) few of which found to be corresponding to peaks in plant extracts spectra. There appear to be no peaks in the amide I and amide II regions characteristic of proteins/enzymes that have been found to be responsible for the reduction of metal ions using microorganisms such as bacteria and fungi for the synthesis of AgNPs. The observed peaks are characteristic of flavanones and terpenoids that are very abundant in Rhododendron plant extracts [28,29]. The peaks observed for AgNPs at 3418 cm-1 (-OH structural polymeric association), 2920 cm-1 (chelating compounds), 1384 cm-1 (germinal methyls), 1076 cm-1 (ether linkages) and 569 cm-1 (C=C groups or from aromatic rings) suggest the presence of flavanones or terpenoids adsorbed on the surface of MNPs. Such interactions between MNPs and plant constituents have been reported [30] in literature. It seems that the oxidation of aldehydic groups of terpenoids in the molecules to carboxylic acids causes the simultaneous reduction of silver salt followed by capping on the surface of synthesized nanoparticle. These findings are supported by the observations reported by Shankar et. al. [31].
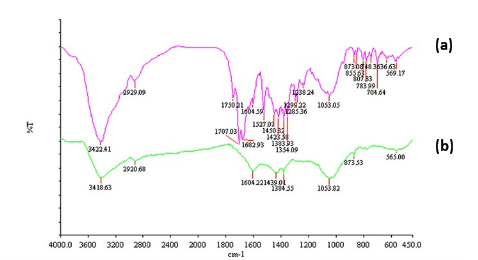
Fig. 5 FTIR spectra overlay of (a) flower extract of R. dauricum and (b) synthesized AgNPs capped with flower constituents
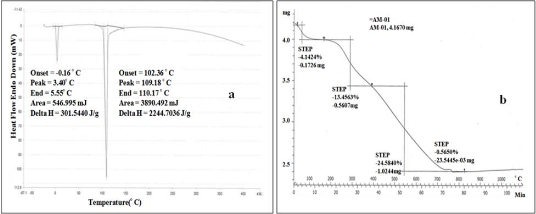
Fig. 6 (a) DSC and (b) TGA analysis of AgNPs synthesized by flower extract of R. dauricum
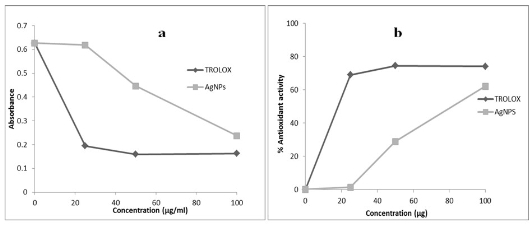
Fig. 7 (a) Scavenging capacity (b) % anti-oxidant activity of the AgNPs as determined by DPPH assay using TROLOX as a standard
3.6. DSC and TGA analysis
DSC study shows the nature of the plant extract compounds loaded on the NPs. Different compounds show their particular characteristic endothermic peaks in DSC. The endothermic peak of AgNPs was found at 100-110 oC (Fig. 6a) due to transition temperature (Tg). These findings are in agreement with the literature report [32]. The TGA plot of the capped AgNPs prepared using Rhododendron dauricum flower extract (Fig. 6b) showed a steady weight loss in the temperature range of 0-100 oC due to moisture loss. Between 100-750 oC weight loss was due to the degradation of organic compounds. Thereafter, above 750 to 1100 oC there was no degradation and remaining content accounts for silver weight. TGA analysis revealed that starting material was 4.16 mg and total loss of organic matter was 1.81 mg (35%).
3.7 DPPH assay analysis
Plants contain specific metabolites that are acknow- ledged to perform a range of purposeful activities. It is well known and also reported in literature that plant mediated nanoparticles synthesis involves sequential reduction followed by capping with these constituents of plants [33]. However, interactions of these components with metals are less well studied and kind of activity they may confer to nanoparticles hasn’t been reported so far. We are reporting for the first time antioxidant activity of silver nanoparticles capped with plant constituents possessing free radical scavenging activity. Antioxidant activity of AgNPs is shown in terms scavenging capacity and % anti-oxidant activity in Fig. 7a and 7b, respectively. Capped silver nanoparticles were found to be potent free radical scavenger when compared to standard TROLOX. Free radical scavenging activity of silver nanoparticles at higher concentration (100 μg mL-1) is comparable to TROLOX. An antioxidant works in stopping the oxidation by neutralizing the free radicals produced. In order to neutralize the free radicals, the antioxidant itself undergoes oxidation. The activity and stability of the silver nanoparticles are affected during antioxidation process and also they are oxidized in the presence of air. This antioxidant activity may be due to the capping constituents present in plant extract and present on metal surface.
4. Conclusions
Synthesis of nanoparticle is basically a reduction process. This reduction is carried out by chemical or biological reducing agent. In the biological process also the reduction maybe by microbes or its extract or may be by plant extract. Whatever may be the reductant, mode of action is same, only reducing agent differs. In the case of plant extract terpenoids, flavonoids and other phenolic compounds are responsible in the synthesis of nanoparticles, whereas in the case of microbes, short chain peptides or proteins may be involved.
Rhododendron dauricum mediated synthesis of silver nanoparticles approach is a fast, green and economical method which produces highly stable AgNPs. Plant is known to contain flavonoids, phenolic, tri-terpenoids and coumarins compounds that seem to play important role in synthesis, stabilization of AgNPs. The synthesized nanoparticles have antioxidant activity due to capped phenolic compounds and can be used against deleterious effects of free radicals.
Acknowledgements
The authors AKM and AK gratefully acknowledge the financial support from the CSIR and DBT, respectively, New Delhi, India.
References
Copyright:(c) 2012 A.K. Mittal, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.