Article
Novel Selective Sensors Based on TiO2 Nanotubes Supported MS(TiO2@MS, M=Cd, Zn) for Their Gas Sensing Properties
Hong Li1, Yingge Zhang1*, Weiping Huang2**
Institute of Pharmacology and Toxicology and Key Laboratory of Nanopharmacology and Nanotoxicology, Beijing Academy of Medi- cal Sciences, Beijing 100850 P. R. China1*, Department of Chemistry, Nankai University, Tianjin 300071, P. R. China2**
*Corresponding authors. E-mail: zhangyg58@126.com; huangw@eyou.com
Citation: H. Li et al., Novel Selective Sensors Based on TiO2 Nanotubes Supported MS(TiO2@MS, M=Cd, Zn) for Their Gas Sensing Properties. Nano Biomed Eng. 2010, 2(2): 143-148.
DOI: 10.5101/nbe.v2i2.p143-148
Abstract
The novel sensors based on TiO2 nanotubes-supported MS (TiO2@MS, M = Cd, Zn) are synthesized via a simple wet chemical method at room temperature. The products are characterized with transmission electron microscopy (TEM), X-ray diffraction (XRD), and photoluminescence spectrum (PL). Their optical and morphological properties indicate the interaction between the TiO2 nanotube and MS nanoparticle. The gas-sensing properties of the obtained samples are studied. Sensors based on the TiO2@MS core/shell heterostructures all show improved gas-sensing properties. The results reveal that the improved performance of the nanocomposites strongly depends on the specific interaction between MS and support.
Keywords: TiO2 nanotubes; Metal sulfide; Core/shell heterostructures; Gas-sensing properties
1. Introduction
The monitoring of toxic and flammable gases has become more important in both domestic and industrial environments. The development in this specific impor- tant technological field requires the invention of relia- ble and inexpensive gas sensors with high sensitivity. TiO2 nanotubes have been introduced as new gas sen- sors with improved sensitivity compared with colloidal or other forms of titania [1-5]. Their high surface to volume ratio and nano-porous structure can offer a large surface area, which bestows them high gas- sensitivity since the interaction of a gas with a metal oxide semiconductor is primarily a surface phenome- non [3–5]. Besides, they are nanosized single crystal- line (so expected to be more stable) with identical crystalline faces exposed to gases, allowing a complete depletion from charge carriers. Meanwhile, it has been reported that the photocatalytic self-cleaning ability [6] of TiO2 nanotubes are also related to their sensitivity to surrounding environment. All the distinct properties of TiO2 nanotubes mentioned above give them great promise for highly sensitive gas sensor applications and, therefore, the synthesis of TiO2 nanotubes with high specific surface for gas sensing elements has been extensively studied [12,13]. Kasuga et al. [14] prepared TiO2 nanotubes by the hydrothermal treatment of TiO2 powders in 10 M NaOH solution in 1998, which is a cheap one-step reaction requiring neither expensive apparatus nor special chemicals with low cost. This method can offer further scope for the practical application of TiO2 nanotube-based sensors. However, TiO2 nanotubes alone are far from ideal gas-sensors. Indeed, gas sensors based on TiO2 nanotubes have been recently reported to show good sensing properties only to H2 [7,8], NO2 [9] and water vapor [10,11]. Except for detection of limited gases, TiO2 nanotubes-based sensing materials have other properties far from satisfactory, including long response time, relatively low gas sensitivity and selectivity. Obviously, continuing efforts should be put into developing new TiO2 nanotubes-based sensing materials to overcome these shortages for better sensors with more satisfactory performances and applications in real environments. Composite type sensors are suggested to improve sensor properties since they contain superior performance than their single-component counterparts in many applications because of their modified physical and chemical properties [15]. Among such nanocomposite structures, CNT/SnO2 and SnO2/CdS core/shell heterostructures have been produced and exhibited enhanced gas sens- ing properties from single phase materials [16,17]. In our previous work, a novel composite structure of TiO2@MS (M = Cd, Zn) was prepared by a wet- chemical method [18] and was revealed a considerable synergetic effect between MS and the support (TiO2 nanotubes) composite catalysts. In this paper, we report their gas sensing properties for the first time.
2. Experimental
2.1. Materials
All the reagents are analytical grade and used with- out any further purification.
2.2. Synthesis
Preparation of MS: Pure MS nanoparticles are prepared according to the literature [19]. The preparation of CdS or ZnS nanoparticles (the process) is the same as our previous work [18]. Preparation of TiO2@MS: The TiO2 nanotubes are synthesized with the method initially developed by Ka- suga et al [14], and then are calcined at 200 ◦C for 1 h. The TiO2@MS core/shell nanostructures are synthe- sized by a similar method reported in our previous work. Briefly, purified TiO2 nanotubes are ultrasonically are dispersed in dry THF, which contains appropriate S powder and stoichiometric CdCl2. Then excess KBH4 is gradually added under vigorous stirring at ambient temperature. After the mixture is stirred for 12 h, TiO2@CdS nanocomosites are formed as a light yellow precipitate. Similar process is carried out to obtain white TiO2@ZnS nanocomposites. The proportions of CdS or ZnS to the total weight of catalysts are 10, 20 and 30 %.
2.3. Characterization
The samples are characterized using transmission electron microscopy (TEM, Philips T20ST) equipped with EDXA capabilities, and X-ray diffraction (XRD, diffractometer with Cu Kα radiation). Photolumines- cence (PL) spectra are collected with a Sperx-F212 luminescence spectrometer.
2.4. Gas sensing properties test
The samples used for the gas sensing studies are prepared as follows: Alumina substrate tube with 4 mm length is used for the heater and sensing base. A Ni-Cr alloy meander heater (outer diameter 0.5 mm) is placed inside the alumina tube to produce an optimized operation temperature. The sensors are fabricated by printing the slurry, which is made by mixing the prepared nomaterials with a 1wt.% aqueous solution, on the electroded substrate. After deposition of the sensing layers, the devices are soldered onto a commercial TO8 socket and hosted in a suitable test chamber. The sensors are heated at different operating temperatures by supplying a given voltage to the heating element. The gas sensing behavior is investigated by using the com- mercial gas sensing measurement system of HW-30A. The measurement is carried out at various concentrations at the range of operating temperature from 100 to 300 ◦C. In this study, C2H5OH (ethanol), NH3 (ammo- nia), C3H6O (acetone), and H2S (sulfureted hydrogen) are used as the detecting gas. The sensitivity (S = Ggas/Gair) of these devices is defined as the conductance variation in air (Gair) and in target gases (Ggas).The response and recovery times are obtained by the time for 90% of total sensitivity change.
3. Results and Discussion
3.1. Characterization of the modified TiO2 nano- tubes
Fig. 1 depicts the XRD patterns of as-prepared TiO2 nanotubes, pure CdS, ZnS nanoparticles and their nanocomposites. The as-prepared TiO2 nanotubes (Fig. 1a) can be indexed to an anatase TiO2 (JCPDS No. 211272). In Fig. 1b, five distinctive TiO2 peaks are found at 25.43◦, 37.92◦, 48.09◦, 54.58◦, 62.81◦ corresponding to (101), (004), (200), (105) (211) and (204) crystal planes, respectively. Also, some additional diffraction peaks corresponding to the hexagonally structured CdS phase (JCPDS Card No. 75-1545) appear. The prominent peaks at angles (2 θ) of 25◦, 28◦, 45◦, 48◦ and 53◦are indexed to reflection from the (100), (101), (110), (103) and (112) planes of hexagonal CdS, respectively. The broad nature of the CdS XRD peaks shows that the sizes of the CdS nanoparticles are very small and the powder has a high degree of crystallinity. The diame- ters of these CdS nanoparticles are about 8 nm (esti- mated from the Scherrer formula). For the Fig. 1d, except the peaks of anatase TiO2, the residual three diffraction peaks marked ring correspond to the (111), (220) and (311) reflections of ZnS, which are completely consistent with the data of the standard card (JCPDS No. 5-0566). In addition, the well-defined peaks in Fig. 1d and e indicate that the formed ZnS is of a high crystallinity. The calculated particle size of ZnS is 3 nm from XRD patterns using Debey-Scherrer formula. Fig. 2 depicts the TEM images of as-prepared TiO2 nanotubes and coated TiO2 nanotubes with different concentrations of the precursors, which has been investigated previously. From the TEM results, a clear distinction between the as-prepared TiO2 nanotubes (Fig.2a-b) and TiO2 nanotubes coated with MS nanoparticles(Fig. 2c-h) can be observed. Representatively, Fig.2c-e shows the selected images of TiO2 nanotubes coated by uniform CdS nanoparticles. From these images, we can see that the amount of CdS particles on TiO2 nanotubes can be readily controlled by changing the concentration of formed S2- ions. Fig. 2c and d display that TiO2 nanotubes are coated with CdS nanoparticles, and partially transform into coaxial nanotubes. Fig. 2e shows that the TiO2 nanotube is coated completely with CdS nanoparticles. These imply that a homogeneous coverage of TiO2@MS nanotubes is obtained by increasing the concentration of precursors. As shown in Fig. 2 f-h, the morphology properties of ZnS nanoparticles coated on the TiO2 nanotubes are similar to that of TiO2@CdS. Along with our previous works [18], a multi-point growth mechanism is proposed to understand the discontinuously and continuously formed MS sheath on the TiO2 nanotubes observed from the TEM images. Eventually, we can get the morphology of MS-coated TiO2 nanotubes which can be changed by adjusting the concentrations of the precursors. Corresponding EDX analysis of TiO2@MS shown in Fig. 3 indicates that the chemical composition of the heterostructure nanotubes is O, Ti, S, Cd or Zn, respectively. These data further confirm that the products are TiO2@MS composite nanotubes. Elemental analysis reveals the atomic percentages of Cd and S are 8.2 and 6.9; Zn and S are 1.9 and 1.4, respectively, which is consistent with the stoichiometric MS within experimental error. Fig. 4 displays the PL spectra of the CdS nanoparticles and the nanocomposites, respectively. The PL spectrum of the CdS nanoparticles (Fig. 4a) shows a sharp emission peak with a maximum around 435 nm, which can be attributed to the emission from the excitonic state near the absorption edge of the particles. Meanwhile, the absence of emission from stokes- shifted trap states suggests the stoichiometric nature of CdS nanoparticles, without a surface excess of Cd2+ or S2- vacancies [20]. On the other hand, since the conduction band of TiO2 lies more positive than that of the CdS conduction band, electron injection is expected from the photoex- cited CdS quantum dots (QDs) into the TiO2 conduc- tion band. The quenching of Q-CdS with the addition of TiO2 must occur when Q-CdS adheres to TiO2 nano- crystals [21]. As expected, the PL spectrum of CdS is entirely quenched for the TiO2@CdS (Fig. 4d), indicat- ing efficient charge injection from the photoexcited CdS QDs into the conduction band of the TiO2 nano- tubes. The increase in quenching of emission with the increase of CdS amounts (Fig. 4b-d) clearly indicates it to be a consequence of increasing interactions between CdS and TiO2 in the nanocomposites. This gives the TiO2@CdS nanocomposites more electrons or holes, respectively, and increases dramatically the conduc- tance of TiO2. Accordingly, it can be inferred that an interaction exists between the coated metal sulfides and TiO2 nano- tube support, and the gas sensing properties of TiO2@MS should be enhanced remarkably.
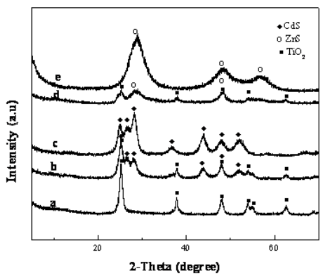
Figure 1. The XRD patterns of catalysts (a) the as- prepared TiO2 nanotubes, (b) TiO2@CdS with 10 wt.% CdS, (c) CdS nanoparticles, (d) TiO2@ZnS with 10 wt.% ZnS, and (e) ZnS nanoparticles.
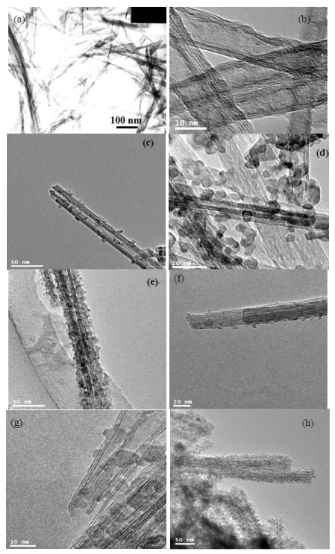
Figure 2. TEM images of samples (a), (b) as-prepared TiO2 nanotubes; TiO2@CdS with different CdS concentrations (c) 10 wt.% (d) 20 wt.% (e) 30 wt.%; TiO2@ZnS with various ZnS concentrations (f) 10 wt.% (g) 20 wt.% (h) 30 wt.%
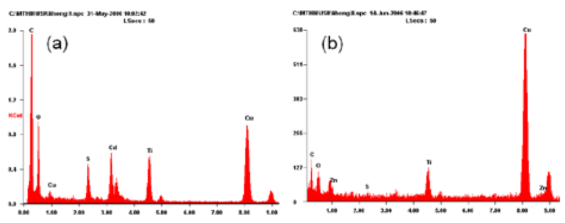
Figure 3. EDX analysis (a) TiO2@CdS with 10 wt.% CdS, (b) TiO2@ZnS with 10 wt.% ZnS.
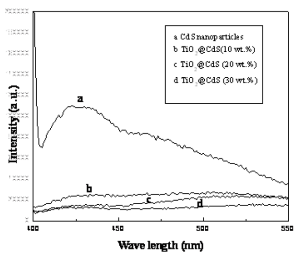
Figure 4. PL spectra of (a) pure CdS nanoparticles, and TiO2@CdS with different concentrations of CdS (b) 10 wt.%, (c) 20 wt.%, (d) 30 wt.% (The excitation wave- length for the emission spectra is 375 nm).
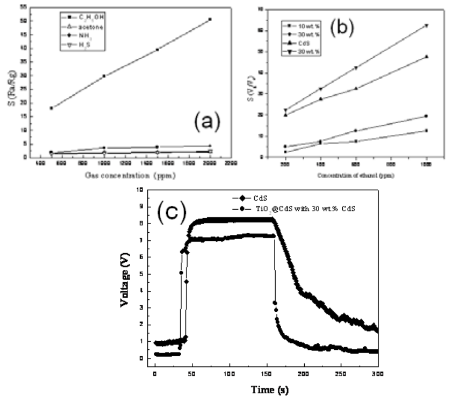
Figure 5. Gas properties of pure TiO2 nanotubes, CdS nanoparticles and their nanocomposites with different concentration of CdS. (a) The relation of sensitivity and gas concentration for various gases, (b) Response– recovery characteristics of the CdS and CdS/TiO2(30%) for1000 ppm ethanol at 230 oC, (c) The relationship between sensitivity of samples and the concentrations of ethanol.
3.2. Gas-sensing properties of the prepared products
The experimental results of the gas sensing properties tested are discussed in detail as follows: Fig. 5a-c and Fig. 6a-c are two gas sensitive systems, respectively. The pure TiO2 nanotubes fail to act as gas sensor in the presence of reducing gases. In comparison with pure CdS nanoparticles, enhanced gas sensing proper- ties of the TiO2@CdS heterostructure nanotubes can be observed in Fig. 5a-c. It is evident from Fig. 5b that CdS is beneficial for the sensitivity of TiO2 nanotubes to ethanol gas, and the sensitivity increases with incre- ment in concentration of CdS up to 30 wt.%. From Fig.5a, sensors based on TiO2@CdS heterostructure nano- tubes (30 wt.%) is the most sensitive to ethanol among the nanomaterials produced, outperforming the responsiveness of pure CdS nanoparticles when operating at 230 °C (Fig. 6c). This experiment result is consistent with the conclusion of Fig. 4. Also, the TiO2@ZnS composites feature an improved H2S-sensing perfor- mance compared to the ZnS nanoparticles (Fig. 6a-c). As shown in Fig. 6a, sensor based on TiO2@ZnS (30 wt. %) is the best sensitivity to H2S at an operating temperature of 160 °C. Typical responses curve of TiO2@ZnS composites gas sensors is presented in Fig.6c, a significantly fast response time of the sensorbased on is observed when exposed to 100 ppm H2S. According to the above tested results, the improvement of the gas sensing activity should be primarily attributed to the coupling efficiency of TiO2 nanotubes and MS, which can be explained by the mechanism of core/shell structures based on a change of electrical conductance [16,17]. In a thermal equilibrium of a TiO2@CdS heterojunction at a reducing atmosphere, electrons from the external CdS shell layer will flow into the inner TiO2 nanotube cores, forming an accumulation layer of electrons in the potential well adjacent to the interface of TiO2@CdS. The electrons injection from excited CdS into the low-lying conduction band of TiO2 results in high concentration of electrons in conduction band of TiO2 nanotubes. Consequently, a new thermal equilibrium at the interface of the TiO2@CdS heterojunction can be achieved. For TiO2@ZnS and ZnS, due to the consequence of the ion-exchangable nature of the TiO2 nanotubes structure, the Zn2+ ions surface-modified TiO2 nano- tubes facilitate the charge separation, which results in a further improvement on the gas-sensing properties for testing H2S in air [22,23]. Considering the efficient charge separations in TiO2@MS core/shell heterostructures, we suggest that the MS nanoparticles would be served as additional electron sources that can greatly improve the electron conduction in TiO2 nanotubes. These additional elec- trons can enhance further the conductance of TiO2, re- sulting in an improvement of the gas sensitivity of TiO2@MS composites in comparison with that of pure MS nanoparticles. Furthermore, the form of the nanotube structures is crucially important to obtain highly dispersed MS par- ticles and sensors with good performance. In this paper, TiO2 nanotubes prepared by hydrothermal treatment [14] exhibit surface areas of 428 m2/g, 100~150 nm in length, and a large one dimensional mesopore/macropore. It indicates that larger surface area and pore size of nanotubes might be beneficial for the gas diffusion and play a crucial role in their short response time, likewise.
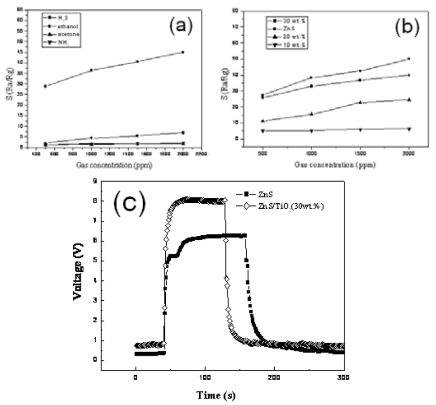
Figure 6. Gas properties of pure TiO2 nanotubes, ZnS nanoparticles and their nanocomposites with different concentration of ZnS. (a) The relation of sensitivity and gas concentration for various gases, (b) Response recovery characteristics of the different element to 1000 ppm H2S at 160 oC, respectively. (c) The relation- ship between sensitivity of samples and the concentrations of H2S.
4. Conclusions
In this paper, the novel sensors based on TiO2@MS heterostructure nanotubes are prepared via a simple wet chemical method at room temperature. The nanocomposites can exhibit higher sensing properties than MS nanoparticles. It is found that the interaction between the TiO2 nanotubes and the MS nanoparticles greatly influences the optical and gas sensing properties of the TiO2@MS composites. These results demonstrate that the core/shell nanostructures can be used as gas sensing materials for fabricating high-performance gas sensors.
Acknowledgments
This work is supported by 973 Program (2005CB623607, 2006CB933304, 2006CB705602 and 2010CB933904) and 863 Program (2007AA021803).
References
Received 20 May, 2010; accepted 18 June, 2010; pub- lished online 12 July, 2010.
Copyright: (c) 2010 H. Li et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.