Anticancer Activity of Copper-Chitosan Nanocomposite Conjugated with Folic Acid
Ghufran K. Ibadi1, Ali A. Taha2, Selma M. H. Al-Jawad3*
1Biomedical Engineering Department, University of Technology, Iraq, Baghdad, Iraq
2Biotechnology Department, School of Applied Sciences, University of Technology, Iraq, Baghdad, Iraq
3Applied Physics Department, School of Applied Sciences, University of Technology, Iraq, Baghdad, Iraq
*Corresponding author. E-mail: selma.m.aljawad@uotechnology.edu.iq; salma_aljawad@yahoo.com
Received: Jul. 16, 2022; Revised: Nov. 26, 2022; Accepted: Dec. 30, 2022; Published: Dec. 31, 2022
Citation: G.K. Ibadi, A.A. Taha, S.M.H. Al-Jawad. Anticancer activity of copper-chitosan nanocomposite conjugated with folic acid. Nano Biomedicine and Engineering, 2022, 14(4): 317–328.
DOI: 10.5101/nbe.v14i4.p317-328
Abstract
Folic acid (FA) is used as target drug delivery for cancer cells by adding to copper/chitosan nanocomposite (Cu/CNC) to form FA-Cu-CS complex. Chitosan is a promising polymeric that has received a lot of attention recently. Chitosan nanoparticles have wide applications as a nanocarrier for different organic and inorganic substances. In the present study, chitosan nanoparticles (CNP), Cu/CNC, and FA-Cu-CS had been prepared and characterized. Structural, morphological, optical and anticancer activities of the nanoparticles were studied. X-ray diffraction study showed the formation of CNP, Cu/CNC, and FA-Cu-CS. FE-SEM showed the CNPs have spherical shape. While, FE-SEM image of Cu/CNC has aggregated and semi-spherical shapes, but the image of FA-Cu-CS showed aggregated, agglomerated, and irregular shapes. FTIR analysis resulted peak at 1026.13 cm–1 refer to the CNPs bonded with folic acid. UV/VIS spectroscopy showed absorbance peak at 530 nm due to presence of FA-Cu-CS. Moreover, anticancer activity of CNPs, Cu/CNC and FA-Cu-CS was tested by MTT assay against fibroblast, MCF-7 and ovarian cancer, then it was stained by DAPI to show the morphology of nucleus. FA-Cu-CS has higher cytotoxicity from other nanoparticles which was 54%, 65% and 83% against fibroblast, MCF-7 and ovarian cancer, respectively at concentration of 0.175 μL/mL. To our knowledge, there is no report on enhancing the anticancer activity of copper/chitosan nanocomposite by conjugated with folic acid and comparison of anticancer activity among CNPs, Cu/CNC and FA-Cu-CS.
Keywords: Copper/chitosan nanocomposite; Folic acid; MTT assay; Anticancer activity; Cytotoxicity of complex FA-Cu-CS; XRD; FE-SEM; UV/VIS spectroscopy
Introduction
Cancer is a dangerous disease that is widespread in the world and causes death. The treatment and prevent of this disease effectively depend on the presence of nanoparticles as a novel anticancer drug. Low cost of nanoparticles and produced in environmentally friendly ways compared with chemotherapy and radiotherapy made them a drug of choice. Moreover, the toxic effects of chemicals and radiations on cancerous cells also revealed the effects on the healthy cells around the target area. Nanoparticles conjugated with different biomedicine as drugs will facilitate the target drug delivery to tumor tissues and control drug release in cancer cell; it does not affect the healthy cells [1–3]. Copper has high melting point, large surface to volume ratio, high thermal conductivity and Plasmon surface resonance (PSR) when it is converted to copper nanoparticles which is responsible for using in many applications, such as killing cancer cells, inhibiting growth of bacteria and toxic effect on most microorganisms [4, 5]. It is transformed to copper nanoparticles (CuNPs) by several methods such as chemical reduction, laser ablation, biological and other methods. It is used in several applications such as anticancer, antimicrobial and others. CuNPs have low stability so that bind with polymers as chitosan for enhancing stability and preventing oxidization [6, 7]. Chitosan is derived from deacetylation of chitin. It is insoluble in water, but it can be dissolved in dilute acid solution. Chitosan nanoparticles (CNPs) have biocompatible, biodegradable and low toxic so that are used in many research [8, 9].
Copper/chitosan nanocomposite (Cu/CNC) is an efficient nanocomposite and it is prepared by coating, encapsulation, biological, chemical reduction, ionic gelation methods. Cu/CNC is operated as antitumor, gene delivery, photothermal, antimicrobial and many applications. Copper/chitosan nanocomposite (Cu/CNC) is better complex than gold/chitoan and silver/chitosan complexes because copper nanoparticles have several features: optical, catalytic properties, excellent conductor of electricity and heat, small size and large surface to volume ratio. It has surface plasmon resonance (SPR) which is responsible for inhibiting growth of microbes and killing cancer cells. While, gold nanoparticles are expensive, toxic, low stable and difficult to synthesis. Moreover, silver nanoparticles are high cost, more volatile, oxidized easily when exposing to air and inhalation of AgNPs causes problems to liver and lungs [10–12]. Folic acid (FA) is tiny ligand used as target drug delivery for cancer cells, which is a promising method to enhance the efficiency and safety of therapeutic agents. FA has high affinity to folate receptors (FR), which are over expressed on the surface of many cancer cells such as ovarian, lung, breast and other cells, while it is less expressed in normal tissues. FA binds with nanoparticles and nanocomposite which can be internalized into cancer cells by receptor-mediated endocytosis [13–15]. In recent years, many types of literature had reported on the synthesis of CuNPs by physical, chemical and biological methods. Zharani et al. studied the preparation of CuNPs by chemical reduction method which is easy and low cost. It produced large quantities of CuNPs with the size of 50–60 nm and spherical shape that was used as anticancer and antimicrobial activities [16]. Cui et al. studied the synthesis of CNPs by ionic gelation method which is low chemical, inexpensive, and simple. Its highly production of CNPs is used as anticancer action [17]. Cheng et al. studied the synthesis of chitosan-folate loaded with ligustrazine which is proved a perfect complex against MCF-7 (breast cancer cell line) [13]. Although review of literature shows the absence of reports for preparing copper/chitosan nanocomposite binding with folic acid (FA-Cu-CS), it is used as anticancer activity. Therefore, the aim of this study is to combine copper and chitosan nanoparticles as a nanocomposite, and characterize by UV-visible spectroscopy, Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), Zeta potential, Field emissionScanning Electron Microscopy (Fe-SEM) and Energy Dispersive Spectroscopy (EDS). Additionally, the improvement of the anticancer activity of copper/chitosan nanocomposite by folic acid is studied.
Experiment Procedures
Preparation of chitosan nanoparticles and copper/chitosan nanocomposite
Chitosan nanoparticles (CNPs) were prepared by dissolving 0.5 g of chitosan in 2% acetic acid under magnetic stirring for 30 min, then add 50 mL of methanol by dropper and 0.05% of Tween80, and stir for 1 h at room temperature to obtain homogeneous solution. After that, 0.05% of sodium tripoly phosphate (STPP) was added gradually to chitosan solution under stirring continuously for 2 h. CuNPs was synthetized by mixing 2 g of starch powder in 100 mL of distill
water with magnetic stirring at 100 ℃ for 30 min and cooled to obtain stabilizing solution, then mixed with mixture of 2 g copper sulfate pentahydrate, 21 g potassium sodium tartrate, and 6 g sodium hydroxides, and stirred at room temperature for 10 min. After that, add 10 mL of formaldehyde under magnetic stirring for 54–60 min until the color of reaction changed from blue to brownish red, indicating the formation of CuNPs. Cu/CNC was synthetized by 0.5 g of CNPs dissolved in 2% acetic acid, then mixed with 0.05 g of CuNPs with magnetic stirring at 80° for 1 h.
Preparation of FA-Cu-CS
0.05 g of folic acid was added to the solution of Cu/CNC and stirred at room temperature for 1 h. The solution was centrifuged and washed 1–3 times until pH reached to 0.7.
Characterization of CNPs, Cu/CNC and FA-Cu-CS
Nano particle and nano composites were characterized by using UV/VIS-specrtophotometry (Schimadzu, Japan) to ensure the formation of samples. Identifing the functional groups in the range of 400–4000 cm–1 was analyzed by FTIR (Schimadzu, Japan). Determining the structure of particles was identified by XRD (Philips, Holanda). The stability and surface charge of samples ware determined by zeta potential (Zetasizer Nano, Malvern, UK). The morphology of samples was measured by FE-SEM (TESCAN, Czech). The chemical elements of nanoparticles were determined by EDS analysis.
Anticancer activity of CNPs, Cu/CNC and FA-Cu-CS by MTT assay
MTT assay was used to identify the cytotoxicity of CNPs, Cu/CNC and Cu-FA-CS complex against cancer cell line (MCF-7, Ovarian cancer) and normal cell line (fibroblast) that were obtained from the Pasteur Institute (Tehran, Iran). Cell lines were grown as monolayer in Dulbeccoʼs Modified Eagle Medium (DMED) supplemented with 10% fetal bovine serum (FBS) and 1% PSF (antibiotic antimycotic solution, penicillin, streptomycin and amphotericin B) in a humidified atmosphere at 37 ℃ with 5% CO2 in 96 well flat-bottom culture plates at density 1×104 cells/well for 24 h. The cells were treated in duplicate with concentrations of 0.075, 0.1, 0.125, 0.15 and 0.175 μL/mL of CNPs. Cu/CNC and FA-Cu-CS complex were incubated for 72 h to determine the toxicity against examined cell lines. 10 μL of MTT solution was added to each well and the plates were incubated for 4 h at 37 ℃. Then, remove the media, and 100 μL of DMSO was added to each well with gentle shaking to dissolve formazan crystals. The absorbance of samples was measured by using ELISA reader at 570 nm. The percentage of cytotoxicity was determined by the equation [18, 19]:
![]() (1)
(1)
where A is the mean optical density (OD) of the control, and B is the mean optical density (OD) of the treated sample.
The apoptosis marker was studied using DAPI stain, after cells were treated with nanoparticles. Cells were stained by DAPI for 1 h, then washed, and used fluorescent microscope at 20× to obtain images [20, 21].
Statistical analysis
The measurements of cytotoxicity were tested in duplicate, and the results were expressed as the mean ± standard deviation. The data were analyzed by two way ANOVA method using SPSS (IBM “SPSS” Statistics - version 26) with a significance probability level of P > 0.05 and P < 0.001 [22].
Results and Discussion
Characterization
X-Ray diffraction analysis
XRD was used to identify the structure of nanoparticle and nanocomposites (crystalline or amorphous). XRD patterns of CNPs showed at angles 2θ = 12.59°, 16.56°, 21.5°, 25.68°, 32.40° and 37.69° corresponding to (300), (600), (110), (210), (103) and (101) which refers to form crystalline CNPs (Fig. 1(a)). This result agrees with Thamilarasan et al [23]. The average crystallitine size of CNPs was 18.65 nm, which was calculated by Scheererʼs equation:
![]() (2)
(2)
where, K = 0.94, λ = 1.5418 nm, β is the full width half maximum (FWHM), and θ is the diffracting angle. Whereas, in the Fig. 1(b), XRD patterns of Cu/CNC appeared slight right shift with abroad peak at 2θ = 25° which indicates to CNPs amorphous structure due to that it acts with CuNPs; while peak at 43° refers to crystalline structure of CuNPs. These result attributed to the CuNPs successfully binding with CNPs as matched with Ebrahimiasl et al. [24, 25]. Finally, the XRD patterns of FA-Cu-CS showed several peaks at angles 2θ = 11°, 21°, 25° and 43° corresponding to the planes (300), (600), (102) and (111) (Fig. 1(c)), which refers to that CNPs have amorphous structure at 25° because it binds with folic acid at 11°, but CuNPs have crystalline structure at 43° which agrees with Ruman et al. who studied synthesis and characterization of chitosan-based nano delivery systems to enhance the anticancer effect [26, 27].
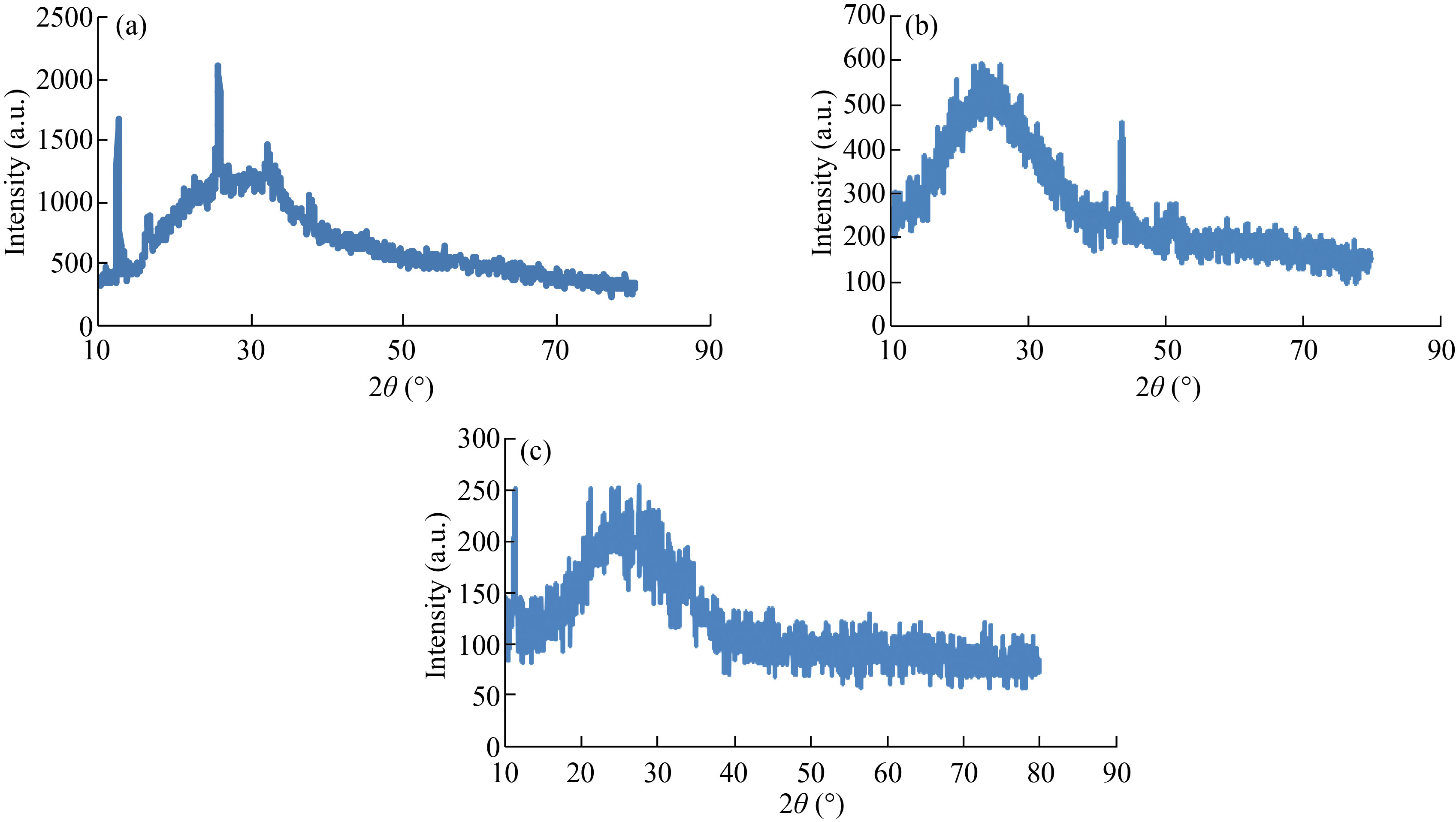
Fig. 1 XRD patterns of (a) CNPs, (b) Cu/CNC and (c) FA-Cu-CS
Fourier transform infrared spectroscopy analysis
FTIR was used to measure the functional groups attached to the surface of CNPs, Cu/CNC and FA-Cu-CS by scanning with wavelength ranging from 400–4000 cm–1. FTIR analysis of CNPs, as shown in the Fig. 2(a), resulted peaks at 1519.91 cm–1, 1543.04 cm–1 and 1627.92 cm–1 due to N–H deformation band, amino and amide groups of CNPs, respectively. Furthermore, peaks at 1319.31 cm–1 and 1215.15 cm–1 indicate to CH2 stretching vibration, 1100–100 cm–1 refers to the saccharide structure of chitosan [12, 28], but FTIR spectrum of Cu/CNC is seen in Fig. 2(b). The peaks at 1400.35 cm–1, 1462.04 cm–1, 1523.76 cm–1, 1543.05 cm–1 and 1651.07 cm–1 were deleted from FTIR of Cu/CNC, but the peaks at 1639.49 cm–1 was decreased to 1635.64 cm–1. 686.66 cm–1 indicates to the combination between CuNPs and CNPs which approved with [28, 29]. Moreover, in Fig. 2(c), FTIR analysis of FA-Cu-CS showed that the peak at 3302.13 cm–1 was attributed to O–H stretching vibration, 1635.64 cm–1 was due to amide band (C=O) of chitosan, 894.97 cm–1 refers to benzene ring of folic acid and 594.08 cm–1 indicates to the bending vibration of Cu–O bond. The band 1026.13 cm–1 refers to the CNPs successfully binding with folic acid that agreed with those in [30–32].
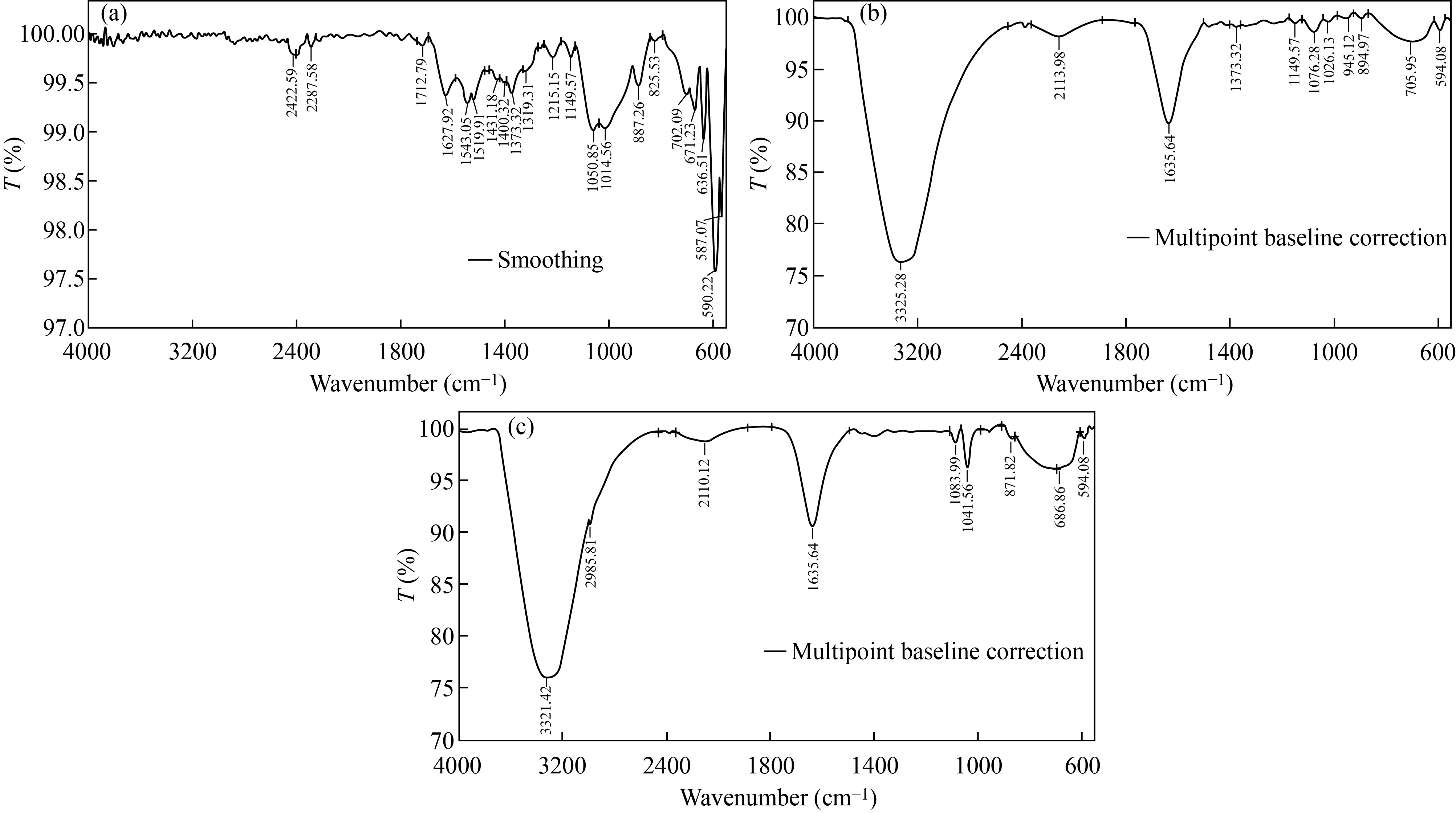 Fig. 2 FTIR spectrum of (a) CNPs, (b) Cu/CNC and (c) FA-Cu-CS
Fig. 2 FTIR spectrum of (a) CNPs, (b) Cu/CNC and (c) FA-Cu-CS
Field emission-scanning electron microscopy
FE-SEM was used to identify the particles size and morphology of CNPs, Cu/CNC and FA-Cu-CS. FESEM of CNPs is spherical shape and highly mono dispersed nanoparticles with the diameter range size of 30–40 nm that agrees with [34], as shown in Fig. 3(a). Whereas, FE-SEM of Cu/CNC showed agglomerated, aggregated and semi-spherical shape with mean diameter of 36.34–48.27 nm, as shown in Fig. 3(b). But in Fig. 3(c), the particles of FA-Cu-CS are aggregated, agglomerated, and irregular shape with the big size of 60–95 nm as a result of added folic acid; this result agrees with [26, 33].
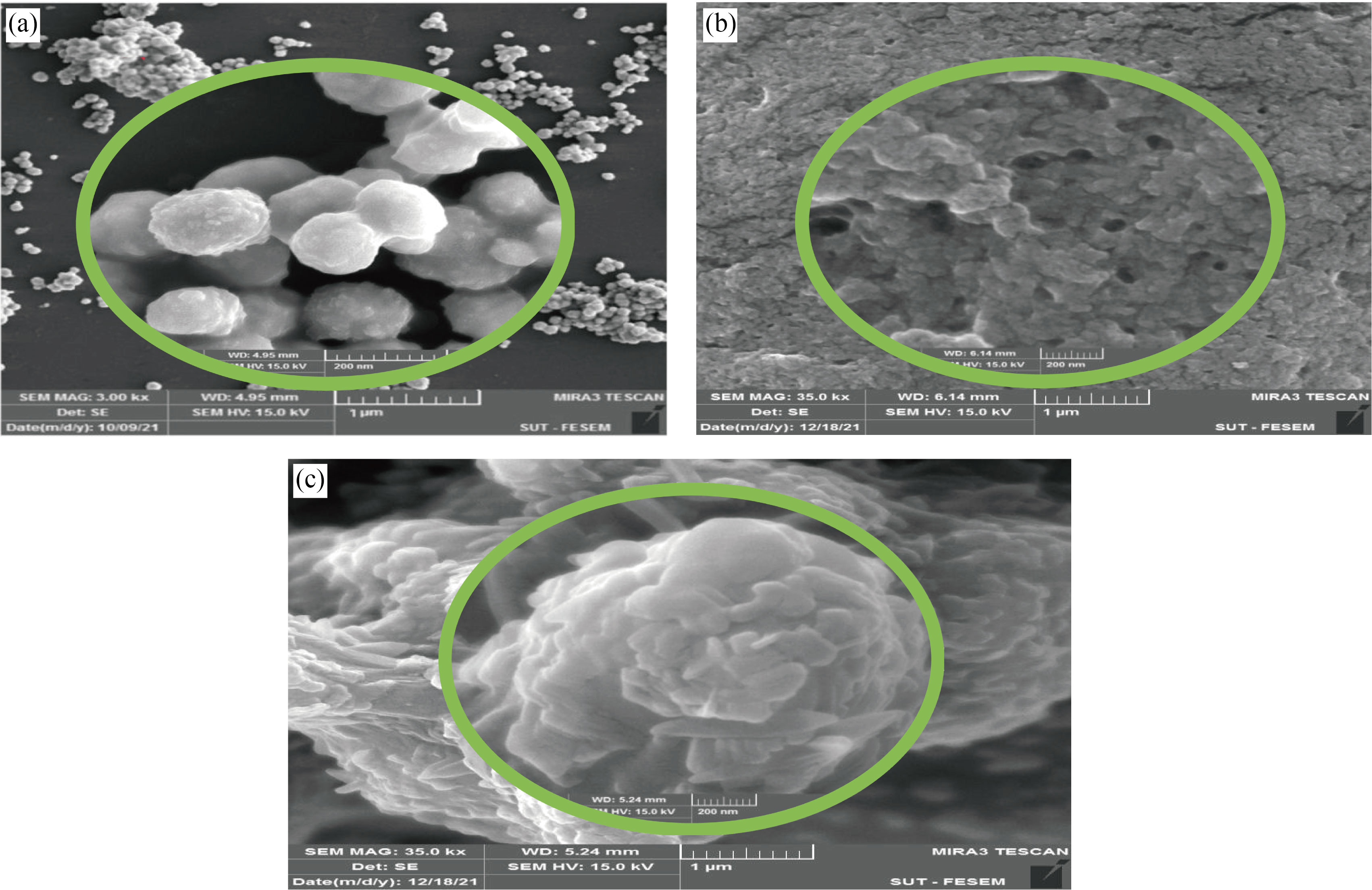
Fig. 3 FE-SEM images of (a) CNPs, (b) Cu/CNC and (c) FA-Cu-CS
Energy dispersive spectroscopy
EDS was used to identify the chemical elements of nanoparticle and nanocomposites. Figure 4(a) showed the EDS of CNPs peaks of C, O, N, P, Na, Mg, Si and Ca. Nitrogen peak indicates to the chitosan formation and agrees with [34], while EDS of Cu/CNC revealed peaks of C, O, Cu, N, and P. Appear of nitrogen refers to CNPs combined with CuNPs to form Cu/CNC and agree with [35], as shown in Fig. 4(b). In Fig. 4(c), the EDS of FA-Cu-CS showed peaks of Cu, C, O, N and P. The presence of nitrogen refers to chitosan. C, O refer to the presence of folic acid because the chemical formula of FA (C19H19 N7O6) and this result agree with [26].
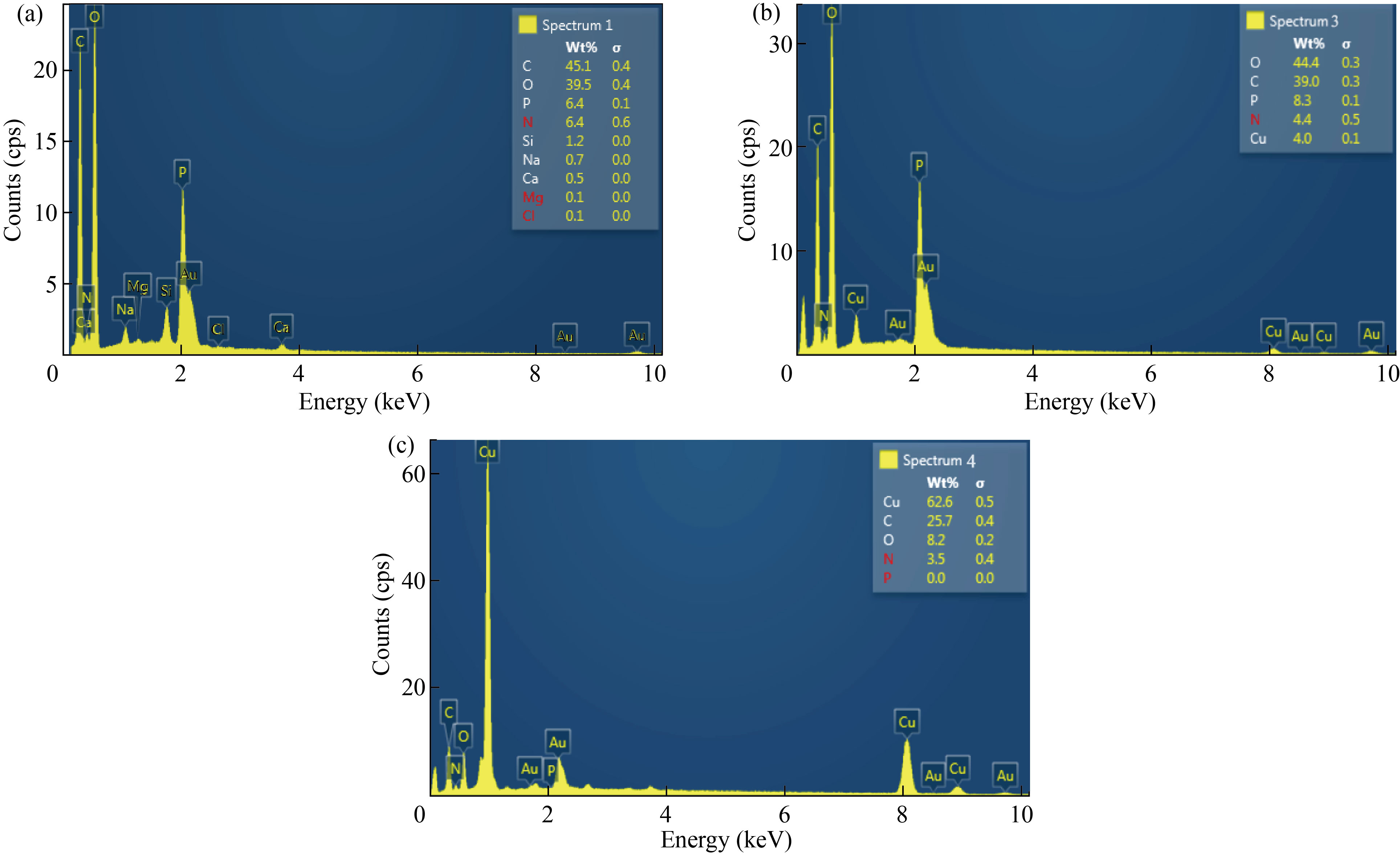
Fig. 4 EDS analysis of (a) CNPs, (b) Cu/CNC and (c) FA-Cu-CS
Ultraviolet-visible spectroscopy analysis
UV-Vis spectrophotometer was used to measure the absorbance of CNPs, Cu/CNC and FA-Cu-CS. The absorption peak of CNPs at 223 nm and no another optical peak appeared, which refers to pure CNPs and agrees with [36], as shown in Fig. 5(a). But absorption peak of Cu/CNC at 550 nm indicates to chitosan successfully combined with copper, which agrees with [4, 37], as shown in Fig. 5(b). The absorption peak of FA-Cu-CS complex appeared at 530 nm, as shown in Fig. 5(c). The decrease of the absorbance indicates folic acid interaction with CS/CNC because the absorption peaks of pure folic acid appeared at 255 nm, 283 nm and 365 nm. This result agrees with Bandara et al. who studied the preparation of zinc/chitosan-folic acid complex [38, 39].
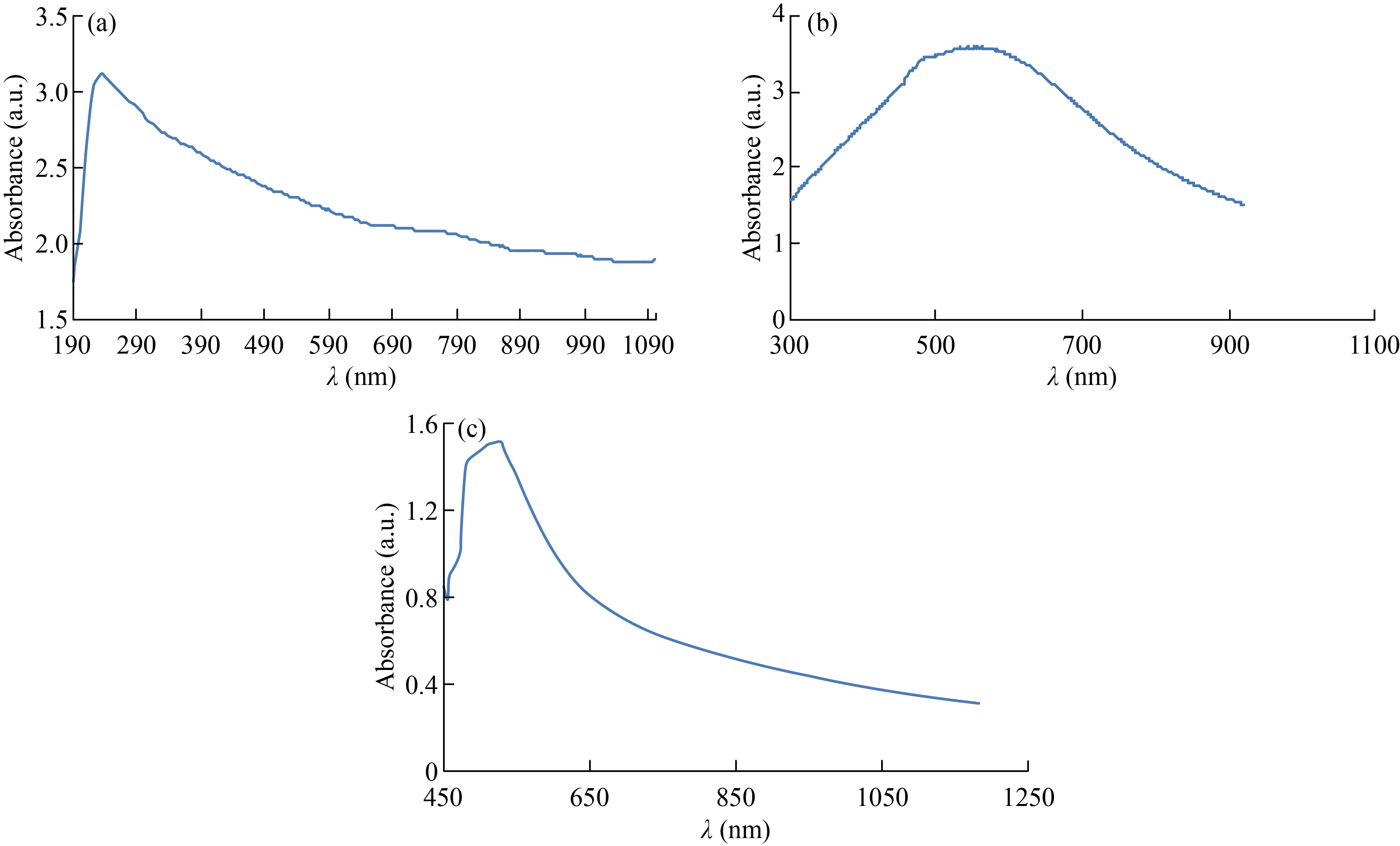
Fig. 5 UV/ V is spectrum of (a) CNPs, (b) Cu/CNC and (c) FA-Cu-CS
Zeta potential analyzing
Zeta potential (ZP) was used to determine stability and surface charge of nanoparticle and nanocomposites. It measured by dynamic light scattering using zeta plus analyzer. This analysis was used at 25 ℃ and after the dispersion was diluted to an appropriate volume with deionized water [40]. The zeta potential value of CNPs was 12.5 mV which refers to the chitosan stable [41], as shown in Fig. 6(a), while the zeta potential value of Cu/CNC was 16.5 mV. The increase in ZP refers to that the CuNPs are more stable in the presence of CNPs, as shown in Fig. 6(b). Moreover, the ZP value of FA-Cu-CS was 14.7 mV, as shown in Fig. 6(c). The decrease in ZP refers to low stability of complex which attributed to folic acid binding with positively charged of chitosan [38].
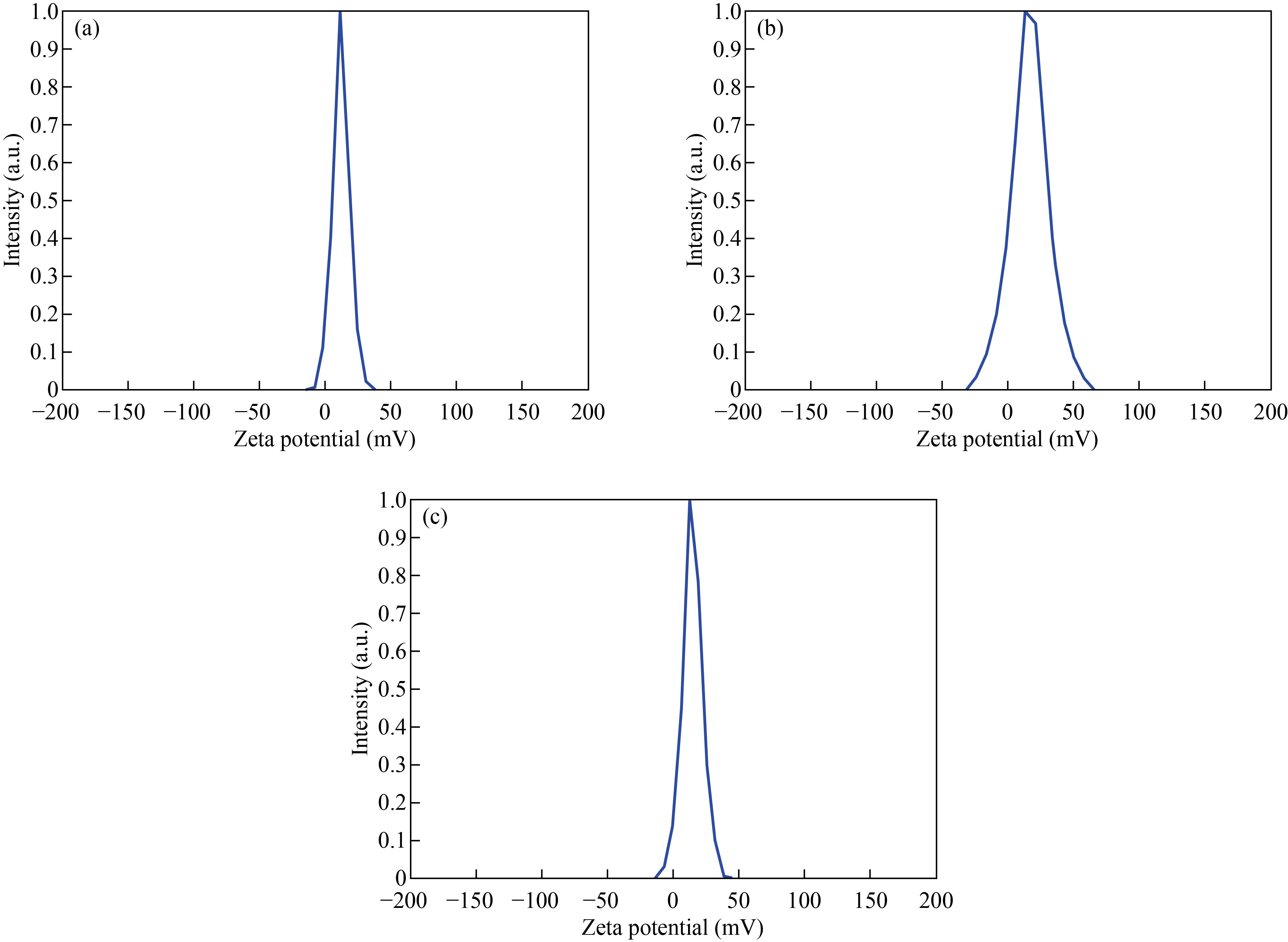
Fig. 6 Zeta potential of (a) CNPs, (b) Cu/CNC and (c) FA-Cu-CS
Anticancer activity of nanoparticle and nanocomposites by MTT assay
MTT assay was used to measure the cytotoxic effect of CNPs, Cu/CNC and FA-Cu-CS against fibroblast; MCF-7 and ovarian cancer cells were incubated for 72 h at 37 ℃ with CO2, as shown in Figs. 7–9. In Fig. 7, the CNPs at concentration of 0.075 μL/mL showed low cytotoxicity against fibroblast; MCF-7 and ovarian cancer were (15 ± 0.4)%, (20 ± 0.7)% and (26 ± 1.1)%, respectively. But it increased gradually to arrive (44 ± 1.98)%, (57 ± 2.85)% and (70 ± 2.3)% at concentration of 0.175 μL/mL. These results refer to the ability of CNPs to form reactive oxygen species (ROS), where the increase concentration of CNPs leads to the increase formation of ROS which causes cell death and low in cell viability. Moreover, when comparing the cytotoxic effect of CNPs against normal cell line with other cancer cell lines, the CNPs showed less cytotoxicity against fibroblast than MCF-7 and ovarian cancer which refers to chitosan safe material that the Food and Drug Administration (FDA) has restricted from using as therapeutic agents [42–44].
On the other hand, in Fig. 8, Cu/CNC appeared at concentration of 0.075 μL/mL with low cytotoxicity to the fibroblast; MCF-7 and ovarian cancer were (12 ± 0.5)%, (20 ± 0.7)% and (52 ± 0.95)%, respectively. But it increased gradually to reach (48 ± 1.5)%, (64 ± 2.1)% and (73 ± 3.1)% at concentration of 0.175 μL/mL. The diversity of the results attributed to the capable of Cu/ CNC to produce ROS that causes oxidative stress to the cancer cells that lead to decease in the cell viability [45–47].
As shown in Fig. 9, FA-Cu-CS revealed that the highest cytotoxicity against MCF-7 and ovarian cancer cell at concentration of 0.175 μL/mL was (65 ± 1.98)% and (83 ± 6.1)%, respectively. But it decreased to (37 ± 1.2)% and (62 ± 2.2)% at concentration of 0.075 μL/mL. Moreover, FA-Cu-CS resulted the highest cytotoxic effect on MCF-7 and ovarian cancer than Cu/CNC and CNPs which attributed to the FA that was used as targeting for cancer cell according to the mechanism. FA has high affinity to folate receptor (FR). FA conjugated nanocomposite binds with folate receptors on the surface of cancer cells, then enters to the cells by endocytosis to form endosomes. And because acidic media (pH = 5–5.5) in the cells lead to separate FR from nanocomposite and return to cell surface. But endosomes are degraded by lysosome, leading to release Cu/CNC to the cytoplasm which enters to the nucleus causing damage of DNA and cells were dead by creating ROS [13, 14, 48]. As shown in Fig. 10, the results indicate that the FA-CuCS has higher cytotoxicity and anticancer activity at concentration of 0.175 μL/mL which can be used as anticancer agent.
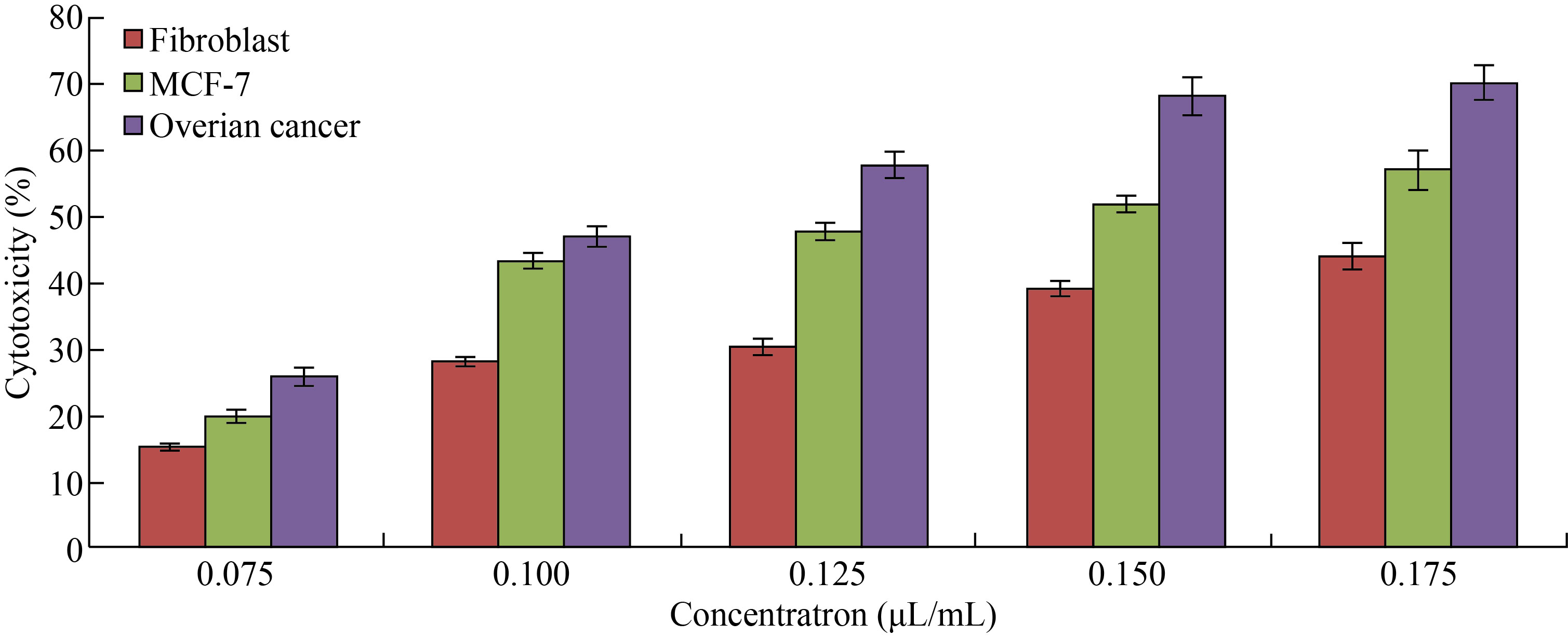
Fig. 7 Cytotoxicity percentage of different concentrations of CNPs against fibroblast, MCF-7 and ovarian cancer cell lines (The values are presented with mean ± SD; P > 0.05 and P < 0.001)
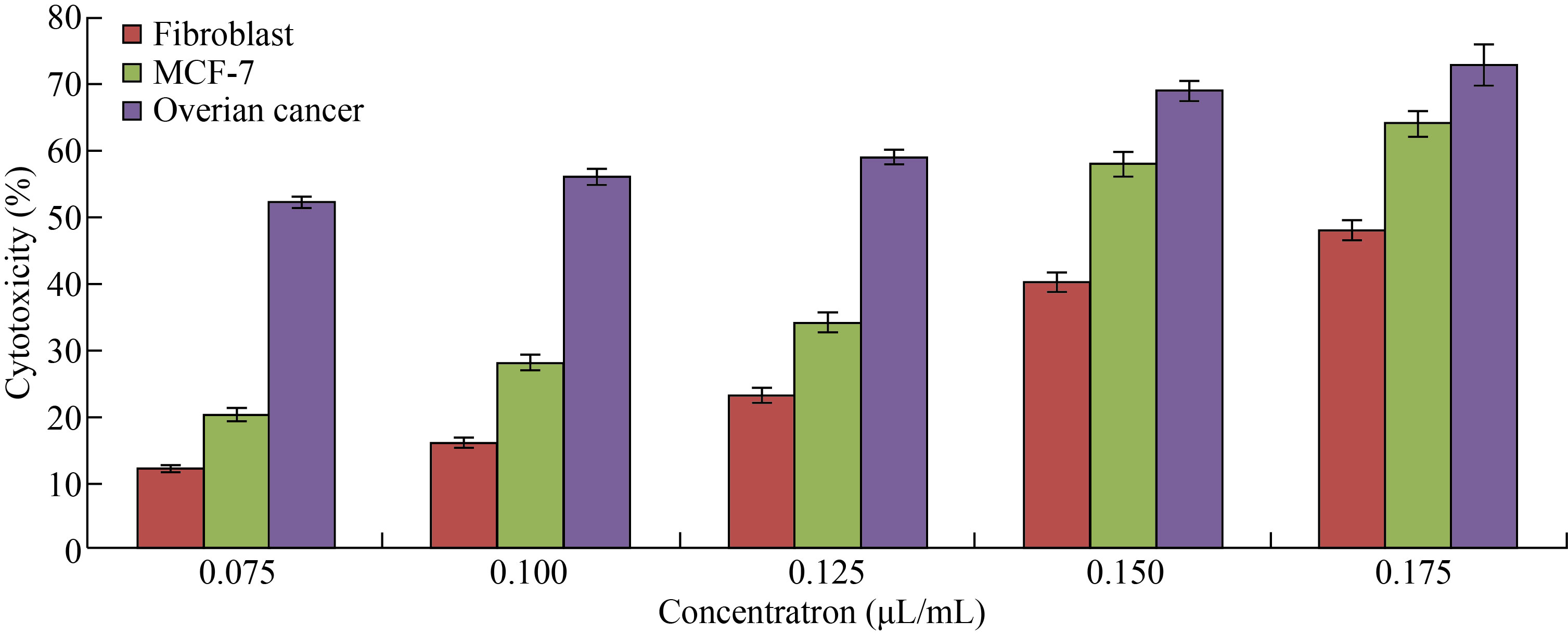
Fig. 8 Cytotoxicity percentage of different concentrations of Cu/CNC against fibroblast, MCF-7 and ovarian cancer cell lines (The values are presented with mean ± SD; P > 0.05 and P < 0.001)
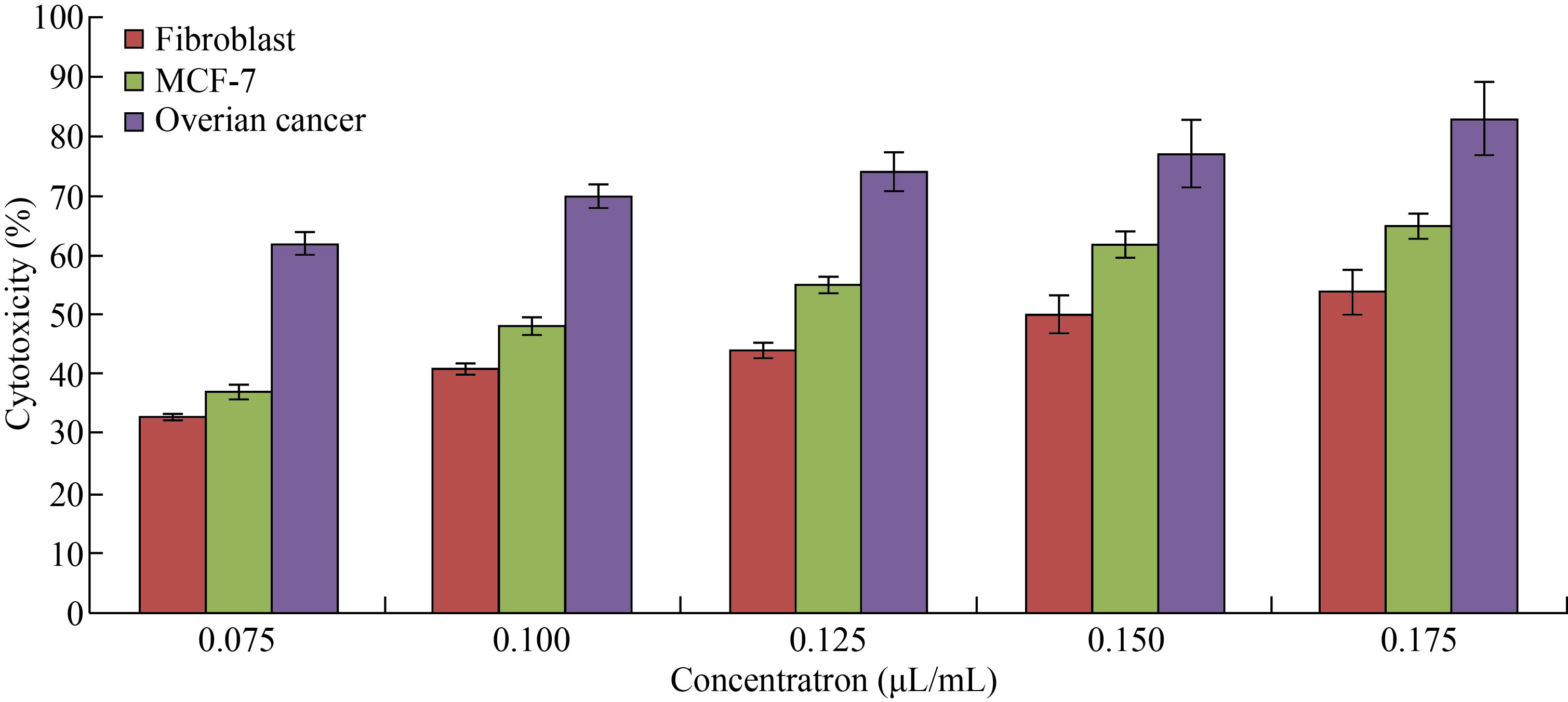
Fig. 9 Cytotoxicity of different concentrations of FA-Cu-CS against fibroblast, MCF-7 and ovarian cancer cell lines (The values are presented with mean ± SD; P > 0.05 and P < 0.001)
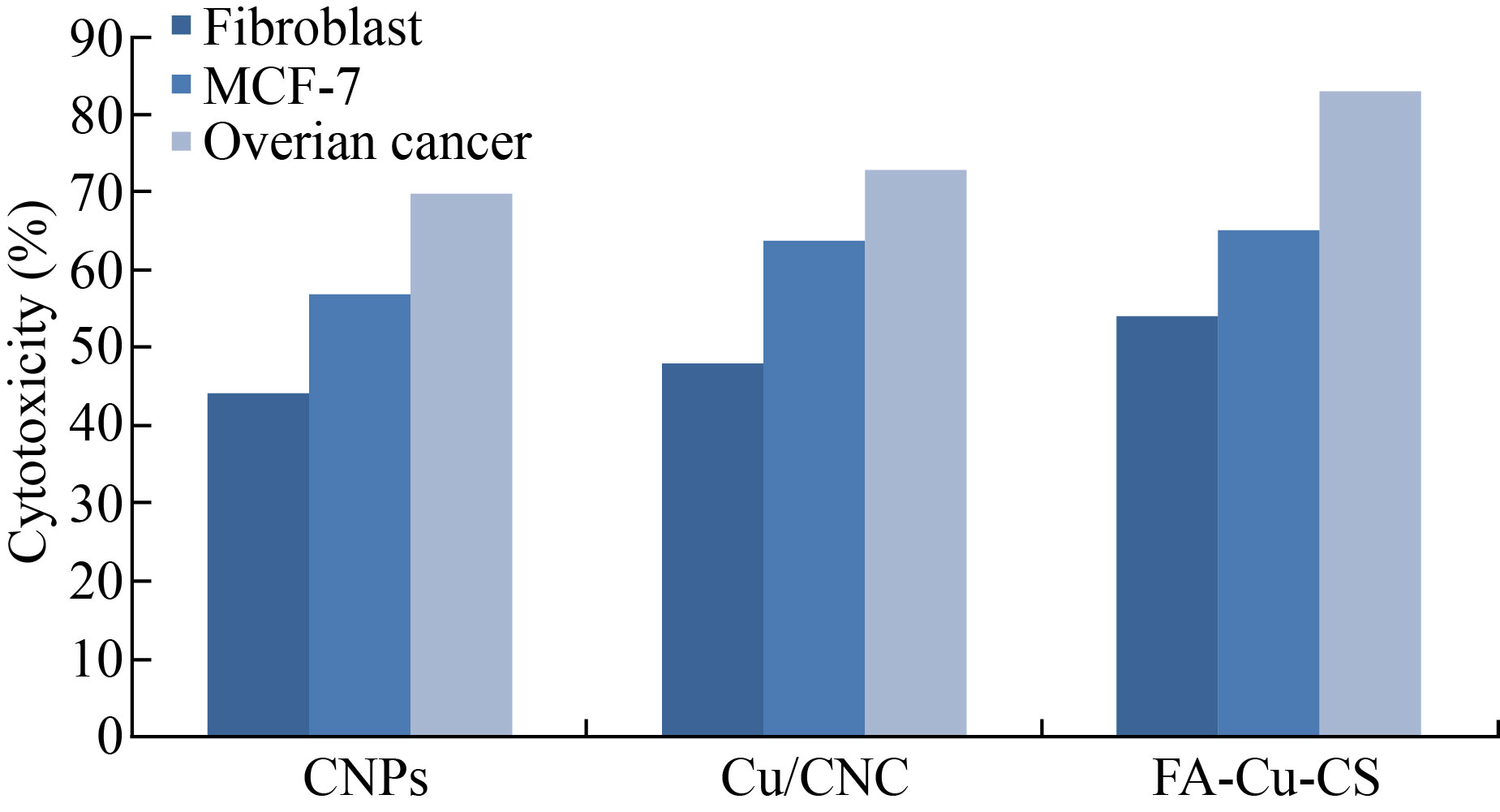
Fig. 10 Cytotoxicity of CNPs, Cu/CNC and FA-Cu-CS against fibroblast, MCF-7 and ovarian cancer cell lines at concentration of 0.175 μL/mL
Cells might phagocytize high content of CNPs, Cu/CNC and FA-Cu-CS produced from intracellular degradation of nanoparticle, and nanocomposites in the acidic media of lysosome could induce cytotoxicity. Figures 11–13 showed the change in the morphology of cell lines after treated with CNPs, Cu/CNC and FACu-CS at concentration of 0.175 μL/mL than untreated cells (control). Nanoparticle and nanocomposites were appeared low changes to fibroblast, but it was appeared higher changes in the MCF-7 and ovarian cancer. The morphological changes include reduction in cell numbers, swelling and shrinkage when compared with untreated cell line that attributed to ROS, damage of DNA, disrupting of membrane and apoptosis process [19, 49, 50]. The inhibition concentration (IC50), the value inhibiting growth of biological activity of cancer cells at 50%, was determined, as listed in Table 1. It refers that IC50 of fibroblast requires more amount of CNPs, Cu/CNC and FA-Cu-CS whose values were 0.16, 0.14 and 0.12 μL/mL for inhibiting growth of it, because it is normal cell line and less or not sensitive with nanoparticle and nanocomposites. Moreover, IC50 of ovarian cancer requires lower amount of CNPs, Cu/CNC and FA-Cu-CS whose values were 0.1, 0.06 and 0.01 μL/mL, because it has higher sensitive to nanoparticle and nanocomposites [51–53].
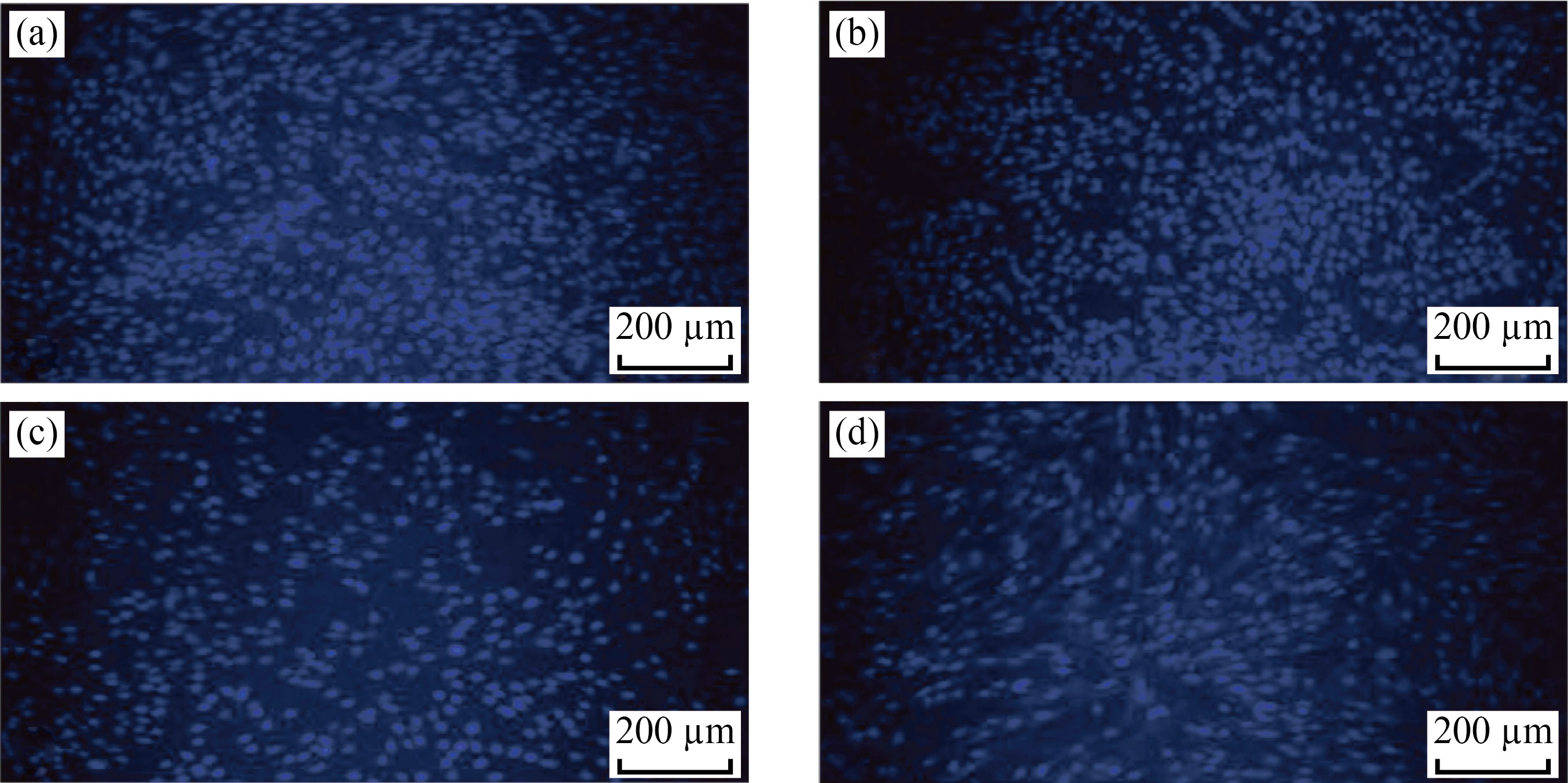
Fig. 11 Fluorescent images of fibroblast cells in the presence of (a) control, (b) CNPs, (c) Cu/CNC and (d) FA-Cu-CS at concentration of 0.175 μL/mL
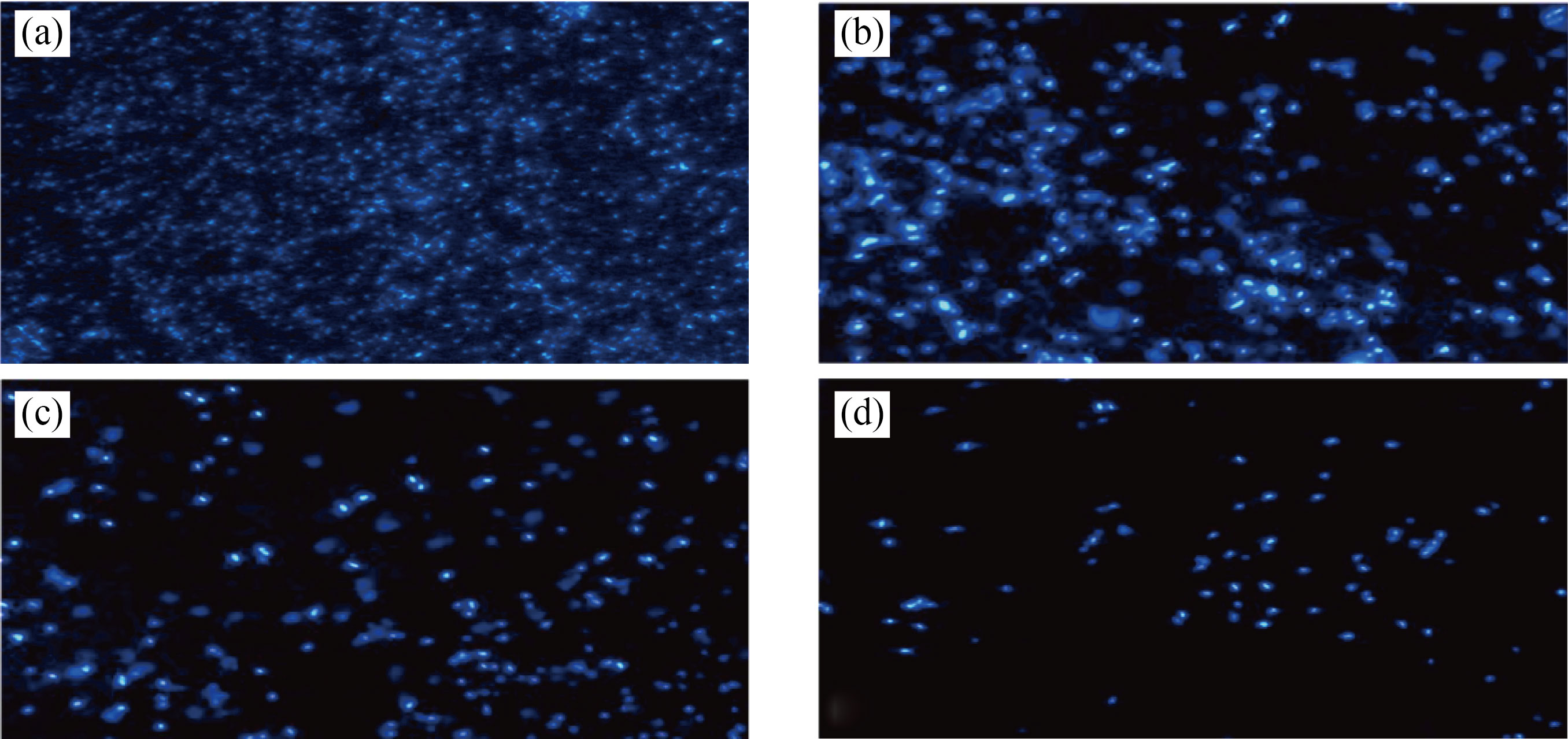
Fig. 12 Fluorescent images of MCF-7 in the presence of (a) control (b) CNPs, (c) Cu/CNC and (d) FA-Cu-CS at concentration 0.175 μL/mL
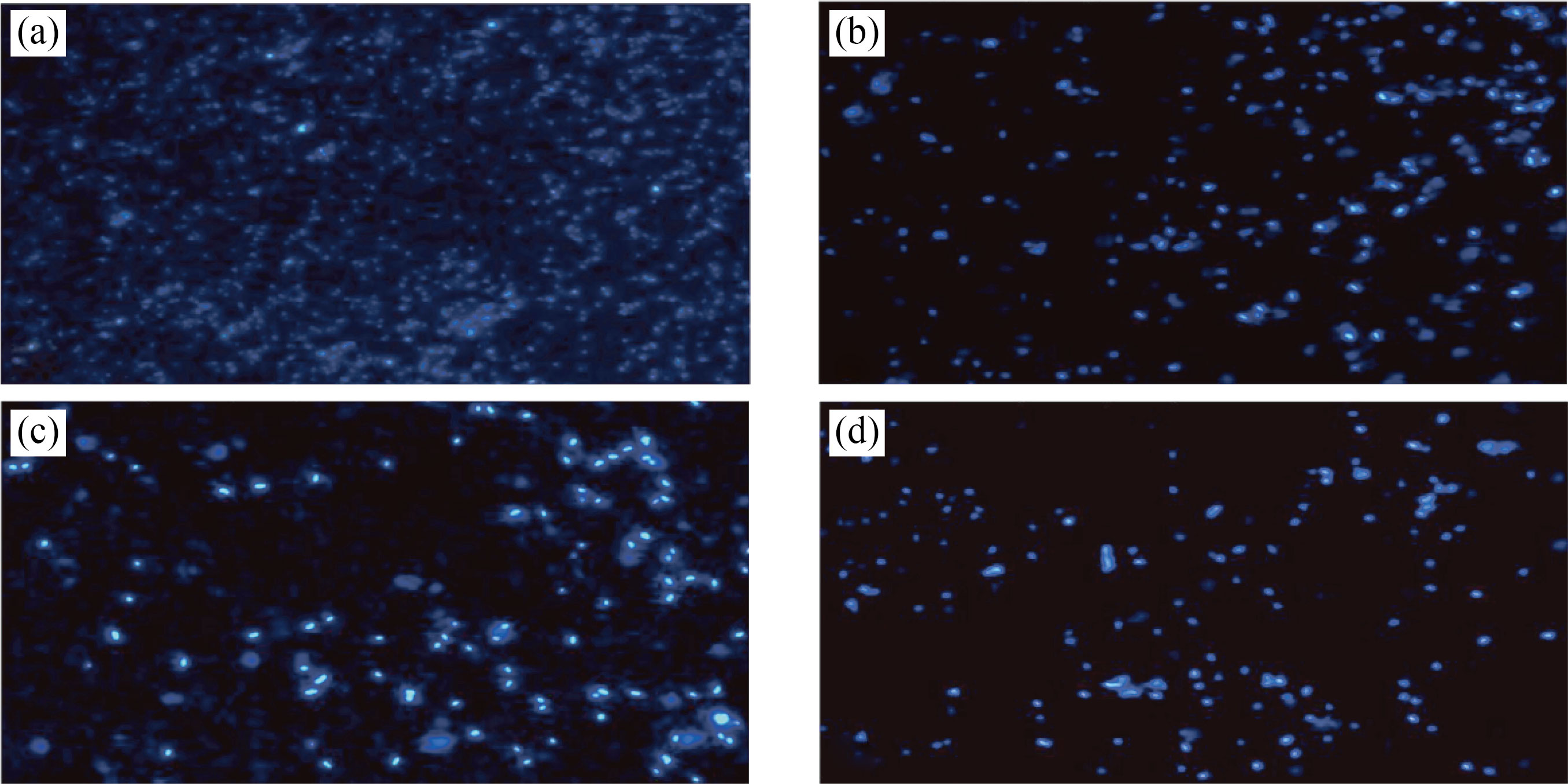
Fig. 13 Fluorescent images of ovarian cancer cells in the presence of (a) control, (b) CNPs, (c) Cu/CNC and (d) FA-Cu-CS at concentration 0.175 μL/mL
Table 1 IC50 values of CNPs, Cu/CNC and FA-Cu-CS
IC50 value (μL/mL) | |||
| CNPs | Cu/CNC | FA-Cu-CS |
Fibroblast | 0.16 | 0.14 | 0.12 |
MCF-7 | 0.14 | 0.12 | 0.11 |
Ovarian cancer | 0.10 | 0.06 | 0.01 |
Conclusion
FA-Cu-CS was successfully prepared by simple and low cost method. FA-Cu-CS showed irregular shape, low stability and amorphous structure. Furthermore, FA-Cu-CS appeared higher anticancer activity than Cu/CNC and CNPs against MCF-7 and ovarian cancer cell lines. Ovarian cancer showed more sensitive to nanoparticle and nanocomposites than fibroblast and MCF-7. FA-Cu-CS resulted high cytotoxicity and anticancer activity at concentration of 0.175 μL/mL which can be used as anticancer agent.
Reference
[1] A. Yaqub, N. Malkani, A. Shabbir, et al. Novel biosynthesis of copper nanoparticles using Zingiber and Allium sp. with synergic effect of doxycycline for anticancer and bactericidal activity. Current Microbiology, 2020, 77(9): 2287–2299. http://dx.doi.org/10.1007/s00284-020-02058-4
[2] A.A. Taha, S.M.H. AL-Jawad, L.F.A. AL-Barram. Improvement of cancer therapy by TAT peptide conjugated gold nanoparticles. Journal of Cluster Science, 2019, 30(2): 403–414. http://dx.doi.org/10.1007/s10876-019-01497-9
[3] S.M.H AL-Jawad, Z.S. Shakir, D.S. Ahmed. Antibacterial activity of Nickel-doped ZnO/MWCNTs hybrid prepared by sol-gel technique. The European Physical Journal Applied Physics, 2021, 96(2): 21201. https://doi. org/10.1051/epjap/2021210115
[4] N. Arjunan, C.M. Singaravelu, J. Kulanthaivel, et al. A potential photocatalytic, antimicrobial and anticancer activity of chitosan-copper nanocomposite. International Journal of Biological Macromolecules, 2017, 104: 1774–1782. https://doi.org/10.1016/j.ijbiomac.2017.03.006
[5] S.M.H. Al-Jawad, A.A. Taha, A.M. Redha, et al. Influence of nickel doping concentration on the characteristics of nanostructure CuS prepared by hydrothermal method for antibacterial activity. Surface Review and Letters, 2021, 28(1): 2050031. https://doi.org/10.1142/S0218625X20500316
[6] M.I. Din, F. Arshad, Z. Hussain, et al. Green adeptness in the synthesis and stabilization of copper nanoparticles: Catalytic, antibacterial, cytotoxicity, and antioxidant activities. Nanoscale Research Letters, 2017, 12(1): 638. https://doi.org/10.1186/s11671-017-2399-8
[7] C. Covarrubias, D. Trepiana, C. Corral. Synthesis of hybrid copper-chitosan nanoparticles with antibacterial activity against cariogenic Streptococcus mutans. Dental Materials Journal, 2018, 37(3): 379–384. https://doi.org/10.4012/dmj.2017–195
[8] Z.S. Shakir, S.M.H. AL-Jawad, D.S. Ahmed. Influence of cobalt doping concentration on ZnO/MWCNTs hybrid prepared by Sol-gel method for antibacterial activity. Journal of Sol-Gel Science and Technology, 2021, 100(1): 115–131. http://dx.doi.org/10.1007/s10971-021-05603-0
[9] A. Ali, S. Ahmed. A review on chitosan and its nanocomposites in drug delivery. International Journal of Biological Macromolecules, 2018, 109: 273–286. https://doi.org/10.1016/j.ijbiomac.2017.12.078
[10] S. Al-Musawi, S. Albukhaty, H. Al-Karagoly, et al. Antibacterial activity of honey/chitosan nanofibers loaded with capsaicin and gold nanoparticles for wound dressing. Molecules, 2020, 25(20): 4770. https://doi.org/10.3390/molecules25204770
[11] I. Salah, I.P. Parkin, E. Allan. Copper as an antimicrobial agent: Recent advances. RSC Advances, 2021, 11(30): 18179–18186. http://dx.doi.org/10.1039/D1RA02149D
[12] R.H. Huseen, A.A. Taha, I.Q. Ali, et al. Biological activity of gum Arabic-coated ferrous oxide nanoparticles. Modern Physics Letters B, 2021, 35(24): 2150411. https://doi.org/10.1142/S021798492150411X
[13] L.C. Cheng, H. Ma, M.K. Shao, et al. Synthesis of folatechitosan nanoparticles loaded with ligustrazine to target folate receptor positive cancer cells. Molecular Medicine Reports, 2017, 16(2): 1101–1108. https://doi.org/10.3892/mmr.2017.6740
[14] S. Houng. Synthesis and characterization of folate-PEG conjugated polysaccharide nanoparticles for potential use as a targeted DNA carrier. Montreal: McGill University, 2009. https://escholarship.mcgill.ca/concern/theses/t435gf61x
[15] X. B. Zhao, H. Li, R. J. Lee. Targeted drug delivery via folate receptors. Expert Opinion on Drug Delivery, 2008, 5(3): 309–319. https://doi.org/10.1517/17425247.5.3.309
[16] M. Al-Zharani, A.A. Qurtam, W.M. Daoush, et al. Antitumor effect of copper nanoparticles on human breast and colon malignancies. Environmental Science and Pollution Research, 2021, 28(2): 1587–1595. https://doi.org/10.1007/s11356-020-09843-5
[17] H.Y. Cui, M. Bai, M.M.A. Rashed, et al. The antibacterial activity of clove oil/chitosan nanoparticles embedded gelatin nanofibers against Escherichia coli O157: H7 biofilms on cucumber. International Journal of Food Microbiology, 2018, 266: 69–78. http://dx.doi.org/10.1016/j.ijfoodmicro.2017.11.019
[18] S.Y. Wang, F. Yan, P. Ren, et al. Incorporation of metalorganic frameworks into electrospun chitosan/poly (vinyl alcohol) nanofibrous membrane with enhanced antibacterial activity for wound dressing application. International Journal of Biological Macromolecules, 2020, 158: 9–17. http://dx.doi.org/10.1016/ j.ijbiomac.2020.04.116
[19] N. Ali, A. Taha, M.K. Mohammed, et al. Mg-doped NiO nanoparticles decorated multi-walled carbon nanotube (MWCNT) nanocomposite and their biological activities. Journal of Nanostructures, 2021, 11(2): 276–285. https://doi.org/10.22052/JNS.2021.02.008
[20]H.K. Al-Salman, E.T. Ali , M. Jabir, et al. 2-Benzhydrylsulfinyl-N-hydroxyacetamide-Na extracted from fig as a novel cytotoxic and apoptosis inducer in SKOV-3 and AMJ-13 cell lines via P53 and caspase-8 pathway. European Food Research and Technology, 2020, 246(8): 1591–1608. http://dx.doi.org/10.1007/s00217-020-03515-x
[21] A.G. Al-Ziaydi, A.M. Al-Shammari, M.I. Hamzah, et al. Newcastle disease virus suppress glycolysis pathway and induce breast cancer cells death. Virus Disease, 2020, 31: 341–348. http://dx.doi.org/10.1007/s13337-020-00612-z
[22] A. Younus, S. Al-Ahmer, M. Jabir. Evaluation of some immunological markers in children with bacterial meningitis caused by Streptococcus pneumoniae. Research Journal of Biotechnology, 2019, 14: 131–133. https://worldresearchersassociations.com/BiotechSpecialIssueMarch2019/20.pdf
[23] V. Thamilarasan, V. Sethuraman, K. Gopinath, et al. Single step fabrication of chitosan nanocrystals using Penaeus semisulcatus: Potential as new insecticides, antimicrobials and plant growth promoters. Journal of Cluster Science, 2018, 29(2): 375–384. http://dx.doi.org/10.1007/s10876-018-1342-1
[24] S. Ebrahimiasl, S.A. Younesi. Shelf life extension of package’s using cupper/(biopolymer nanocomposite) produced by one-step process. Journal of Food Biosciences and Technology, 2018, 8(1): 47–58. https://jfbt.srbiau.ac.ir/article_11257.html
[25] M.A. Jihad, F.T.M. Noori, M.S. Jabir, et al. Polyethylene glycol functionalized graphene oxide nanoparticles loaded with Nigella sativa extract: A smart antibacterial therapeutic drug delivery system. Molecules, 2021, 26(11): 3067. https://doi.org/10.3390/molecules26113067
[26] U. Ruman, K. Buskaran, G. Pastorin, et al. Synthesis and characterization of chitosan-based nanodelivery systems to enhance the anticancer effect of sorafenib drug in hepatocellular carcinoma and colorectal adenocarcinoma cells. Nanomaterials, 2021, 11(2): 497. https://doi.org/10.3390/nano11020497
[27] M.S. Jabir, T.M. Rashid, U.M. Nayef, et al. Inhibition of Staphylococcus aureus α-hemolysin production using nanocurcumin capped Au@ZnO nanocomposite. Bioinorganic Chemistry and Applications, 2022, 2022: 2663812. https://doi.org/10.1155/2022/2663812
[28] M.S. Usman, M.E.E. Zowalaty, K. Shameli, et al. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. International Journal of Nanomedicine, 2013, 8: 4467–4479. http://dx.doi. org/10.2147/IJN.S50837
[29] K.S. Alghamdi, N.S.I. Ahmed, D. Bakhotmah, et al. Chitosan decorated copper nanoparticles as efficient catalyst for synthesis of novel quinoline derivatives. Journal of Nanoscience and Nanotechnology, 2020, 20(2): 890–899. https://doi.org/10.1166/jnn.2020.16923
[30] Vikas, M.K. Viswanadh, A.K. Mehata, et al. Bioadhesive chitosan nanoparticles: Dual targeting and pharmacokinetic aspects for advanced lung cancer treatment. Carbohydrate Polymers, 2021, 274: 118617. https://doi.org/10.1016/j.carbpol.2021.118617
[31] H.H. Bahjat, R.A. Ismail, G.M. Sulaiman, et al. Magnetic field-assisted laser ablation of titanium dioxide nanoparticles in water for anti-bacterial applications. Journal of Inorganic and Organometallic Polymers and Materials, 2021, 31(9): 3649–3656. http://dx.doi.org/10.1007/s10904-021-01973-8
[32] T. Rashid, U.M. Nayef, M. Jabir, et al. Study of optical and morphological properties for Au-ZnO nanocomposite prepared by Laser ablation in liquid. Journal of Physics: Conference Series, 2021, 1795: 012041. http://dx.doi.org/10.1088/1742-6596/1795/1/012041
[33] M.K.A. Mohammed, M.R. Mohammad, M.S. Jabir, et al. Functionalization, characterization, and antibacterial activity of single wall and multi wall carbon nanotubes. IOP Conference Series: Materials Science and Engineering, 2020, 757: 012028. https://doi.org/10.1088/1757-899x/757/1/012028
[34] S. Rafigh, A. Heydarinasab. Mesoporous chitosanSiO2 nanoparticles: Synthesis, characterization, and CO2 adsorption capacity. ACS Sustainable Chemistry & Engineering, 2017, 5(11): 10379–10386. http://dx.doi.org/10.1021/ACSSUSCHEMENG.7B02388
[35] H.F. Zhou, A. El-Kott, A. Ezzat Ahmed, et al. Synthesis of chitosan-stabilized copper nanoparticles (CS-Cu NPs): Its catalytic activity for C-N and C-O cross-coupling reactions and treatment of bladder cancer. Arabian Journal of Chemistry, 2021, 14(10): 103259. http://dx.doi.org/10.1016/j.arabjc.2021.103259
[36] M. Agarwal, M.K. Agarwal, N. Shrivastav, et al. Preparation of chitosan nanoparticles and their In-vitro characterization. International Journal of Life-Sciences Scientific Research, 2018, 4(2): 1713–1720. https://doi.org/10.21276/ijlssr.2018.4.2.17
[37] S. Al-Musawi, S. Albukhaty, H. Al-Karagoly, et al. Dextran-coated superparamagnetic nanoparticles modified with folate for targeted drug delivery of camptothecin. Advances in Natural Sciences: Nanoscience and Nanotechnology, 2020, 11(4): 045009. https://doi.org/10.1088/2043-6254/abc75b
[38] S. Bandara, C.A. Carnegie, C. Johnson, et al. Synthesis and characterization of Zinc/Chitosan-Folic acid complex. Heliyon, 2018, 4(8): e00737. http://dx.doi.org/10.1016/j.heliyon.2018.e00737
[39] M.M. Radhi, H.N. Abdullah, M.S. Jabir, et al. Electrochemical effect of ascorbic acid on redox current peaks of CoCl2 in blood medium. Nano Biomedicine and Engineering, 2017, 9(2): 103–106. https://doi.org/10.5101/nbe.v9i2.p103-106
[40] S.S. Swain, L. Unnikrishnan, S. Mohanty, et al. Synthesis and characterization of surface-functionalized mesoporous graphene nanohybrid. Applied Nanoscience, 2019, 9(7): 1531–1552. http://dx.doi.org/10.1007/s13204-019-00963-0
[41] A.K. Gatta, R. Chandrashekhar, N. Udupa, et al. Strategic design of dicer substrate siRNA to mitigate the resistance mediated by ABCC1 in doxorubicin-resistant breast cancer. Indian Journal of Pharmaceutical Sciences, 2020, 82(2): 329–340. https://doi.org/10.36468/pharmaceuticalsciences.654
[42] S.M.H. AL-Jawad, A.A. Taha, A.M. Redha. Studying the structural, morphological, and optical properties of CuS: Ni nanostructure prepared by a hydrothermal method for biological activity. Journal of Sol-Gel Science and Technology, 2019, 91(2): 310–323. http://dx.doi.org/10.1007/s10971-019-05023-1
[43] R. Resmi, J. Yoonus, B. Beena. Anticancer and antibacterial activity of chitosan extracted from shrimp shell waste. Materials Today: Proceedings, 2021, 41: 570–576. http://dx.doi.org/10.1016/j.matpr.2020.05.251
[44] T. Kean, M. Thanou. Biodegradation, biodistribution and toxicity of chitosan. Advanced Drug Delivery Reviews, 2010, 62(1): 3–11. http://dx.doi.org/10.1016/j.addr.2009.09.004
[45] M. Hasanin, M.A. Al Abboud, M.M. Alawlaqi, et al. Ecofriendly synthesis of biosynthesized copper nanoparticles with starch-based nanocomposite: Antimicrobial, antioxidant, and anticancer activities. Biological Trace Element Research, 2022, 200(5): 2099–2112. https://doi.org/10.1007/s12011-021-02812-0
[46] M.M. Muhsen, S.M.H. Al-Jawad, A. Taha. GumArabic-modified Mn-doped CuS nanoprisms for cancer photothermal treatment. Chemical Papers, 2022, 76: 6821–6838. http://dx.doi.org/10.1007/s11696-022-02364-0
[47] S.M.H. AL-Jawad, A.A. Taha, L.F.A. AL-Barram. Effective cancer treatment by targeted pH sensitive-gold nanoparticles without using laser irradiation. Journal of Sol-Gel Science and Technology, 2019, 89(2): 473–485. http://dx.doi.org/10.1007/s10971-018-4832-6
[48] H.J. Song, C. Su, W.Y. Cui, et al. Folic acid-chitosan conjugated nanoparticles for improving tumor-targeted drug delivery. BioMed Research International, 2013, 2013: 723158. https://doi.org/10.1155/2013/723158
[49] L.F.A. AL-Barram, S.M.H. AL-Jawad, A.A. Taha, et al. Photothermal therapy of cancer cells enhanced by glutathione (GSH) modified small-sized gold nanoparticles. Journal of Electromagnetic Waves and Applications, 2020, 34(18): 2467–2487. https://doi.org/10.1080/09205071.2020.1822759
[50] Z.K. Taha, S.N. Hawar, G.M. Sulaiman. Extracellular biosynthesis of silver nanoparticles from Penicillium italicum and its antioxidant, antimicrobial and cytotoxicityactivities. Biotechnology Letters, 2019, 41(8): 899–914. http://dx.doi.org/10.1007/s10529-019-02699-x
[51] G.M. Sulaiman, M.S. Jabir, A.H. Hameed. Nanoscale modification of chrysin for improved of therapeutic efficiency and cytotoxicity. Artificial Cells, Nanomedicine, and Biotechnology, 2018, 46(S1): 708–720. http://dx.doi.org/10.1080/21691401.2018.1434661
[52] A.A. Taha, S.M.H. AL-Jawad, A.M. Redha. Preparation and characterization of nanostructure CuS for biological activity. Modern Physics Letters B, 2019, 33(30): 1950374. https://doi.org/10.1142/s0217984919503743
[53] S.M.H. Al-Jawad, S.H. Sabeeh, A.A. Taha, et al. Synthesis and characterization of Fe-ZnO thin films for antimicrobial activity. Surface Review and Letters, 2019, 26(5): 1850197. https://doi.org/10.1142/S0218625X18501974
Copyright© Ghufran K. Ibadi, Ali A. Taha and Selma M. H. AlJawad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.