Titanium Dioxide Nanoparticle and Cardiovascular Diseases: A Critical Review of the Literature and Possible Underlying Mechanisms
Shiva Mehran1, Soroush Ghodratizadeh2, Ali Zolfi-Gol3, Hamed Charkhian4,
Mojtaba Ranjbari4, Vahed Ebrahimi5, Zafar Gholinejad6,*
1Department of Biology, Higher Education Institute of Rabe-Rashidi, Tabriz, Iran
2Department of Biochemistry, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran
3Department of Pediatrics Cardiology, Shahid Motahari Hospital, Urmia University of Medical Sciences, Urmia Iran
4Department of Biology, Urmia Branch, Islamic Azad University, Urmia, Iran
5Department of Biochemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran
6Department of Medical Laboratory Science, Urmia Branch, Islamic Azad University, Urmia, Iran
*Corresponding author. E-mail: zafar.gholinejad777@gmail.com; ghzafar@yahoo.com
Received: Jul. 13, 2022; Revised: Nov. 03, 2022; Accepted: Nov. 28, 2022; Published: Dec. 31, 2022
Citation: S. Mehran, S. Ghodratizadeh, A. Zolfi-Gol, et al. Titanium dioxide nanoparticle and cardiovascular diseases: A critical review of the literature and possible underlying mechanisms. Nano Biomedicine and Engineering, 2022, 14(4): 329–342.
DOI: 10.5101/nbe.v14i4.p329-342
Abstract
Background: Over the last decades, the exposure to titanium dioxide nanoparticles (TiO2 NPs) has increased due to the wide application in industry, food adduct, medicine, cosmetic products, etc. Literature review showed that the TiO2 NPs exert toxic effects on several organs.
Methods: We searched PubMed, MEDLINE and the other databases with the following keywords, “titanium dioxide nanoparticle”, “TiO2 NPs”, “myocardial infarction”, “endothelial”, “blood pressure”, “heart” and “cardiovascular”, and reviewed the literature by focusing on the toxic effects of TiO2 NPs on the cardiovascular system, and possible underlying mechanism.
Results: The toxic effects of TiO2 NPs on the cardiovascular system are controversial but some possible mechanisms were proposed. TiO2 NPs and nanoparticle-derived titanium induce cardiac injury, endothelial dysfunction and increase blood pressure and heart rate. These effects are mediated via systemic or local oxidative stress and inflammation.
Conclusion: The TiO2 NPs toxicity is dependent on cell type and particle characteristic, and the controversial results may be due to these variables. However, a growing body of evidence confirmed the possible TiO2 NPs toxicity on the cardiovascular system.
Keywords: Titanium dioxide; Nanoparticle; Cardiovascular diseases; Endothelial dysfunction; Oxidative stress; Cardiac injury; Inflammation
Introduction
Cardiovascular diseases (CVDs) are the major health problem with a high mortality rate in the worldwide [1]. Nanomaterial technology helps the diagnosis and treatment of CVDs while nanomaterial pollution and nanotoxicity lead to CVDs progression [2–4]. It is well-documented that soil, water, food and air pollution increase the risk of CVDs incidence and further mortality [5, 6]. On the other hand, titanium dioxide nanoparticles (TiO2 NPs) are important source of environmental pollution by because its application is frequent in the industry, food adducts, medicine, cosmetic products, etc [7]. As well as, TiO2 NPs occupational exposure occurs during production, bagging and waste manipulation [8].
The pharmacokinetics evidence on TiO2 NPs exposure does not support the penetration of the nanoparticles via skin cosmetic product [9]. Standardized guideline authorities and researchers have no consensus that daily food product exposure could reach the toxic doses [10]. However, experimental study introduces the gastrointestinal tract as a blood source of nanoparticles [11]. There are compelling reports regarding the toxic effect of TiO2 NPs on respiratory system, and pulmonary TiO2 NPs are able to reach the blood circulation and distributed system [12]. One or more transferring proteins mediate the blood transferring of natural hydrophobic molecules. In case of TiO2 NPs, an artificial hydrophobic structure proposes that probably interacts with serum proteins and become de-agglomerated [13, 14]. Due to hydrophobic properties of TiO2 NPs and their aggregation, the amount of penetration and tissue-tissue translocation seem to be at low level, but compared with fining particles, it occurs significantly [15, 16].
Titanium dioxide is a powder phase martial which is safe enough to be considered as a biologically inert chemical with a wide scale uses [17]. The titanium dioxide is manufactured to nanomaterial with a plethora of new properties compared with their fine powder. Titanium dioxide nanomaterials exist in several shapes including nanoparticle with anatase, rutile brookite phases and nanotubes [18]. The current toxicological studies showed that alloys and pure forms of titanium are safe and non-toxic elements for human [19, 20]. However, the properties of materials
are changed at the nano dimension.
Shi et al. reviewed the toxicokinetics information of TiO2 NPs where the blood content of nanoparticles is sourced by skin, gastrointestinal tract, and respiratory system and distribute to the whole of body via blood and circulatory system [21]. A great deal of compelling evidences confirmed that the toxic effects of TiO2 NPs are on lungs, kidneys and reproductive system, which have been reviewed in detail elsewhere [22–24]. However, less attention has been paid to the importance and mechanisms of TiO2 NPs toxicity and environmental exposure in term of CVDs.
Cardiovascular system would be a susceptible organ to the TiO2 NPs toxicity due to several reasons. Previous studies have showed that the TiO2 NPs toxicity is dependent on exposure load (a function of concentration and exposure time), nanoparticle physiochemical characteristics (such as size, surface potential and phase (anatase, rutile and brookite)), and tissue properties (lipid content, metabolism and antioxidant capacity) [25, 26]. The cardiovascular system is exposed to TiO2 NPs at the significant loads because pharmacokinetics studies showed that the blood circulation transports the TiO2 NPs and distributes them to the whole body. Therefore, the cardiac tissue and the endothelium expose to the TiO2 NPs directly. In this term, the cardiovascular system is similar to lung with a huge TiO2 NPs exposure and severe organ damage [27].
Lipid content and metabolism is an integral of cardiovascular system, and it is an essential factor in the susceptibility to oxidative stress which is the main molecular mechanism of TiO2 NPs toxicity [28, 29]. Therefore, the cardiovascular system susceptibility to TiO2 NPs may be due to higher lipids content and metabolism which acts in similar way reported in high lipid content tissue including brain and reproductive organs.
There is consensus that reactive oxygen species (ROS) generation, oxidative stress and further inflammation mediate the TiO2 NPs toxicity. On the other hand, oxidative stress and inflammation
play pivotal roles in the atherosclerosis, endothelial dysfunction, hypertension, cardiac damage and other cardiovascular pathologic events. This is the third reason that cardiovascular system is at the risk of TiO2 NPs toxicity.
Here we reviewed the evidence regarding the cardiovascular system toxicity. We first discussed the direct effects of TiO2 NPs on cardiac tissue including the histopathological change, the titanium content of heart and the post exposure serum levels of cardiac damage biomarkers. Then, we reviewed the relationship between TiO2 NPs, dyslipidemia and atherosclerosis. We focused on endothelial dysfunction, hematological parameters and thrombotic system that paly pivotal roles in CVDs. The molecular mechanisms of TiO2 NPs-induced oxidative stress and inflammation were also discussed. Ultimately, we summarized the effects of TiO2 NPs on blood pressure and heart rate. The mechanisms of TiO2 NPs toxicity were reviewed elsewhere by Hou et al. that the adopted depiction is represented in Fig. 1.
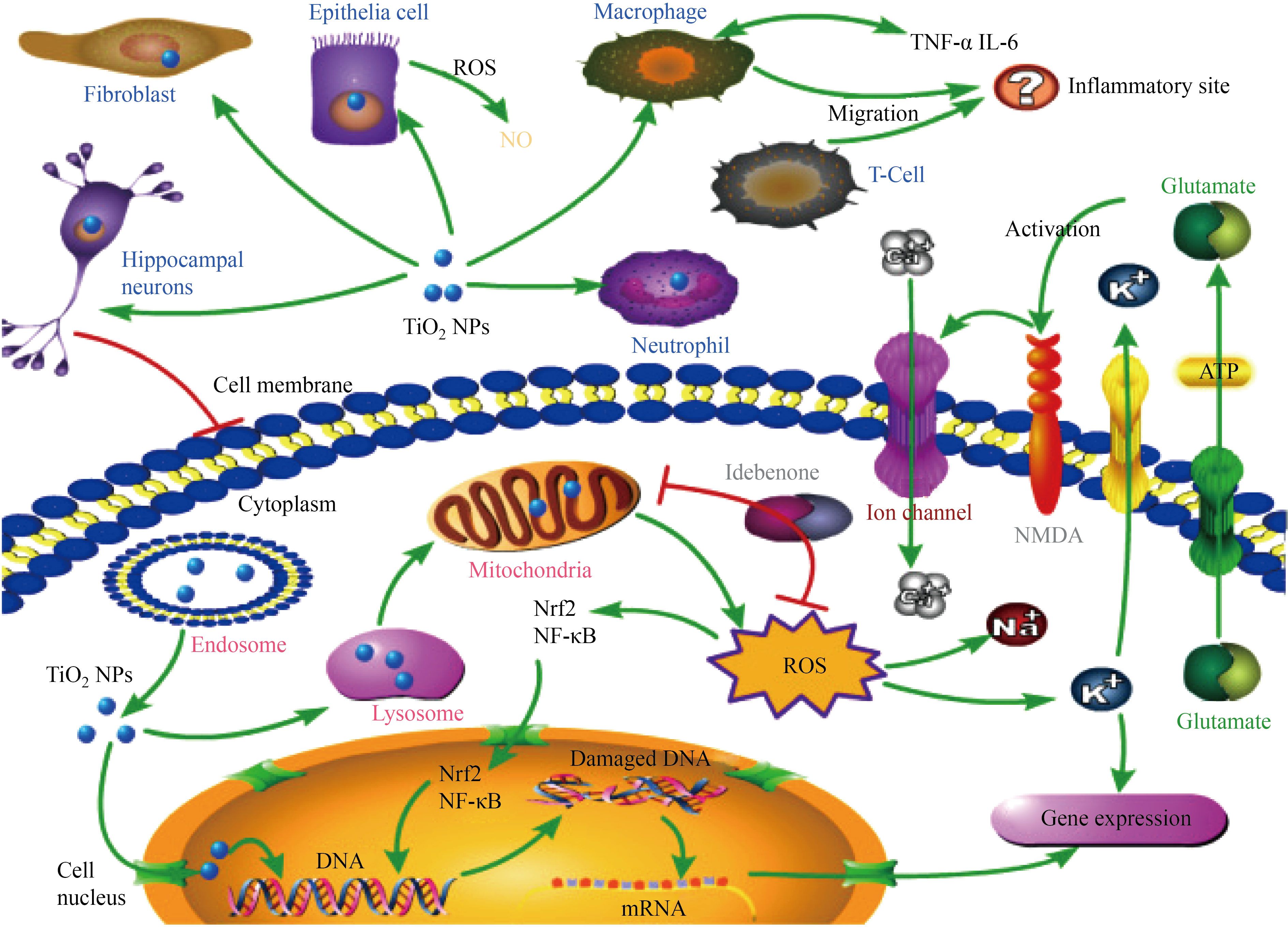
Fig. 1 Hou et al. summarized the toxicity of TiO2 NPs [30]
Methods
We conducted an online searching in PubMed, MEDLINE and the other databases with the following keywords: “titanium dioxide nanoparticle”, “TiO2 NPs”, “myocardial infarction”, “endothelial” blood pressure”, heart” and “cardiovascular”, and reviewed the literature. All the published articles in the aforementioned databases until December 2020 were included to this review and we tried to cover the reports comprehensively.
First, we try to find the direct toxic effect of TiO2 NPs on heart tissue and its pathology. The relationship between nanoparticles, dyslipidemia and atherosclerosis was reviewed and then we focused on endothelial dysfunction as a most reported mechanism. A review of abstracts proposed that the oxidative stress and inflammation were considered deeply as an underlying molecular mechanism but there were articles reported the nitric oxide, and vasodilation disturbance, heart rate, blood pressure, and bio toxic effect of TiO2 NPs. The effect of nanoparticles on vital organs and
development of CVDs as a consequence was reviewed as well.
Results
Heart histopathology and titanium content
After injection, oral administration and pulmonary absorption, the TiO2 NPs are distributed in the spleen, kidney, liver, brain, and other organs. Compared with the skin, gastrointestinal truck, and respiratory system, heart is not the leading tissue in the TiO2 NPs exposure; however, there are articles that showed TiO2 NPs is predisposed in the cardiac tissue [31, 32].
The effects are ranged from titanium predisposing without cardiac damage, elevation of cardiac damage biomarkers without histopathological changes and obvious cardiac damage and biomarker elevation.
Some studies showed that after TiO2 NPs administration, the titanium reaches and penetrates to the heart tissue and mediate cardiac histopathological injury directly. TiO2 NPs internalize to the cardiomyoblasts via actin-ediated endocytosis [33].
Other studies found an alteration in the heart size, histopathology, protein phosphorylation, cardiac injury biomarkers after TiO2 NPs administration. Sheng et al. reported that titanium accumulates in the mouse heart in dose dependent manner after intragastric administration, which leads to cell necrosis, cardiac histopathological changes and elevated creatine kinase (CK) levels [34]. In another study, TiO2 NPs were found in the heart tissue after a single dose intratracheal administration (2 mg/kg TiO2 NPs) which causes to arrhythmia and echocardiographic pattern change in the rat model [35].
Kan et al. showed that the pulmonary exposure to TiO2 NPs causes to the change in the cardiac protein phosphorylation and biosynthesis of substance P, but the effects are independent from systemic inflammation [36]. Chang et al. showed an intratracheal high dose TiO2 NPs (4.0 and 32 mg/kg) administration reduces the coefficients of the heart. However, no significant histopathological change was observed in the heart tissue [37]. In this study, aspartate transaminase (AST), creatine kinase (CK), lactate dehydrogenase (LDH), and α-Hydroxybutyrate dehydrogenase (α-HBDH) increased significantly at the high doses of nanoparticles. In another study, after oral administration of TiO2 NPs (5 g/kg body weight (BW)) serum LDH and alpha-HBDH levels increased but the cardiac histopathological change was not observed [38]. Nemmar et al. showed that the instillation of rutile TiO2 NPs (1 and 5 mg/kg for 24 h) induces the cardiac edema and increases the ratios weight–to–dry weight in the Wistar rats’ heart [39]. The long term nasal administration (6 months) leads to myocardial cell swelling and increases the CK and LDH levels [40].
In contrast to the aforementioned studies, others have provided evidence that shows TiO2 NPs have no direct toxic effects on the heart. Liu et al. showed that abdominal injection of TiO2 NPs (at 5, 10, 50, 100, and 150 mg/kg BW, anatase phase, for 14 days) did not affect coefficients of the hearts and the hearts contains the minimum titanium content among organs [41]. Chen et al. showed that heart titanium content and histopathological changes did not change post nanoparticle exposure [42]. Elgrabli et al. demonstrated that after intravenous injection of TiO2 NPs, distribute TiO2 NPs were in the arterial circulation and end organs, but they could not induce heart damage [43]. Treatment of adult and young rats with 50 mg/kg TiO2 NPs did not induce the histopathological change but affected the heart size in young but not adult rats. This study showed that the elevation of α-HBDH and CK levels in the adults are lower than young rats, indicating that the age affects the susceptibility to TiO2 NP toxicity [44]. Chen et al. reported that daily gastrointestinal administration of TiO2 NPs for 30–90 days has no significant pathological effect on the cardiac tissue compared with the control group [45]. However, the LDH and other cardiac biomarker were changed, suggesting the cardiac injury without obvious histopathological change [45]. Despite the inconsistencies, it is intuitive that TiO2 NPs exposure probably leads to accumulation of titanium in the heart and induces the histopathological change and elevation of serum cardiac injury biomarkers. The toxic effect is a function of administrated dose, exposure time and administration route.
Atherosclerosis and dyslipidemia
Dyslipidemia is the most important risk factor for development of atherosclerosis that leads to CVDs ultimately. Some studies have been carried out to investigate the role of TiO2 NPs on the development of atherosclerosis and dyslipidemia. Yu et al. showed that the exposure to TiO2 NPs increases serum levels of triglycerides, glucose, total cholesterol, low-density lipoprotein cholesterol (LDLc), advanced glycation end products while it reduces high-density lipoprotein cholesterol (HDLc), nitric oxide and tissue plasminogen activator that accelerates the atherosclerotic lesions [46]. Similar results were observed in the earlier study by Chen et al. who have used the ApoE knockout mice [47]. The TiO2 NPs exposure induces a modest plaque progression in the aorta of ApoE knockout rat model. However, the vasodilatory function and expression of genes such as a monocyte chemoattractant protein-1 (Mcp-1), macrophage inflammatory protein 2 (Mip-2), vascular cell adhesion protein 1 (VCAM-1, intercellular adhesion molecule-1 (ICAM-1) and vascular endothelial growth factor (VEGF) did not changed in the lung tissue [48]. In another study, TiO2 NPs cause to dyslipidemia and induce the atherosclerosis and plaque formation in the ApoE knockout mice [49]. Tang et al. performed a metabolomics study on the intratracheal TiO2 NPs treated rats, which showed that postexposure LDLc levels were increased significantly [50]. In Hu et al.’s study, the lipid profile did not alter in the TiO2 NPs-treated mice but glucose metabolism was impaired [51]. Hence, we concluded that diabetes, glucose metabolism impairment, and a pivotal CVDs risk factor may play mediatory roles in the TiO2 NPs induced atherosclerosis and further CVDs [52].
As we know, the conversion of macrophages to the foam cells results in endothelial injury, which is a key step in the CVDs progression. Suzuki et al. showed that TiO2 NPs do not affect the foam cell formation [53]. However, the other studies showed that TiO2 NPs and nanotube could induce some changes in the macrophages cells [54–57].
Endothelial and angiogenesis
Endothelial is the first bulwark in the cardiovascular system that exposes to the toxic materials in the circulation. On the other hand, the endothelial injury and dysfunction are critical steps in the progression of CVDs. As well as, the endothelium plays a pivotal role in the angiogenesis. The zebrafish in vivo model exposure to TiO2 NPs slightly reduces the angiogenesis [55]. Hou et al.’s study on the endothelial cells showed that, TiO2 NPs are much more toxic than SiO2 and Fe3O4 nanoparticles. In spite of lower cell internalization of TiO2 NPs than others, it produces a significant amount of intracellular ROS that reduces the GSH/GSSG ratio (oxidative stress indicator) in time and dose dependent manner. TiO2 NPs internalization, mitochondrial accumulation, oxidative stress, alteration of cytoskeleton and cell morphology and ultimately apoptotic cell death were suggested as a mechanism of TiO2 NPs toxicity [58]. By the way, nanoparticle cell internalization is a critical factor in cell toxicity [59].
García et al. showed that TiO2 NPs could increase the intracellular ROS and cell adhesion capacity, and reduce the cell proliferation [60]. Montiel-Dávalos et al. showed that the human umbilical vein endothelial cells (HUVECs) treatment with TiO2 NPs (20 μg/cm2 ) causes to apoptosis via oxidative stress, NF-κB activation, adhesion proteins change and NO production [61]. Ramos-Godínez et al. introduced a co-culture models to assessment of TiO2 NPs toxicity that showed the adhesion molecules (E-selectin, ICAM-1, VCAM-1) expression and monocyte-HUVECs adhesion are increased significantly. The effects of TiO2 NPs are mediated by nitric oxide and of pro-inflammatory cytokines production [62]. Han et al. showed that the TiO2 NPs increases the superoxide generation, Akt (also called Protein kinase B), extracellular signal-regulated kinases (ERK), Jun N-terminal kinases (JNK) and p38 mitogen activated protein kinases (p38) phosphorylation, NF- κB activation, MCP-1 and VCAM-1 gene expression. In this study, autophagy is introduced as a defense mechanism against TiO2 NPs toxicity but it cannot compensates and reverses the toxic effects completely [63].
TiO2 NPs could localize into the human brain-derived endothelial cells and cause to ROS production, depletion of thiols, slightly reduction of DNA synthesis, DNA damage, and expression of autophagy-lysosomal markers [64]. The cellular internalization of TiO2 NPs leads to adhesion and inflammatory molecules overexpression that follows a cell type dependent manner [65]. We performed a comprehensive experimental study on the toxic effect of TiO2 NPs on HUVECs that confirmed the nanoparticle internalization to the cells. We also showed the nanoparticle induce oxidative stress via intrinsic source such as mitochondrial free radical production. We showed that TiO2 NPs activate PI3K/ Akt and NF-κB signaling pathways and deactivate p38 pathway. As well as, TiO2 NPs lead to lipid peroxidation and cell membrane disruption but have no significant on nitric oxide synthase enzymes [59, 66]. The effect of TiO2 NPs induced-mitochondrial dysfunction as a mechanism of cardiovascular toxicity was confirmed by other studies [67]. Another study on HUVECs showed that the post exposure cell culture media contain the higher LDH activity, total superoxide dismutase levels, NO content, tumor necrosis factor lpha (TNF-α) and Interleukin 6 (IL-6) levels [68]. Peng et al. investigated the response of primary human endothelial and the vascular smooth muscle cells to TiO2 nanotube but not nanoparticle and showed that the nanotubes enhance proliferation and cell motility, and decrease the proliferation vascular smooth muscle cells; a downexpression of molecules inflammatory and coagulation factors is observed in both cell types [69].
However, other studies do not confirm the toxic effects of TiO2 NPs on endothelial cells. In this
regard, Gu et al. showed that TiO2 NPs have no significant cytotoxic effects on HUVECs (treated
with 2–32 μg/mL) without any significant change in secretion of TNF-α and IL-6 levels [70]. TiO2 NPs at the 1–100 μg/mL concentration did not have effect on cell viability but the apoptosis increases when cells were treated with 100 μg/mL concentration. Others found that TiO2 NPs internalize to the cells and cause to nuclear fragmentation but did not affect intracellular ROS and LDH release from microvascular endothelial cells [71]. Spigoni et al. showed that circulating angiogenic cells treatment with TiO2 NPs could not induce oxidative stress and cell death but cellular function was impaired [72]. Evaluation of industrial sources of TiO2 NPs showed that the TiO2 NPs existing in aged paints but not pristine paints reduce the human lung microvascular endothelial cells viability. Total glutathione and ICAM-, TNF-α and IL-6 secretion were not affected by both aged and pristine paints derived TiO2 NPs. but pristine nanoparticles reduce the IL-8 secretion significantly [73]. Suzuki et al. observed that TiO2 NPs (1–100 μg /mL) could not internalize to the HUVECs and exert no toxic effects on the cell viability. They found no significant change in the the MCP-1 production, ICAM-1 and VCAM-1 protein expression, monocytes adhesion assay [53]. Bengalli et al. showed that TiO2 NPs affect the human pulmonary microvascular endothelial cell line (a co-culture model of the airblood barrier) by releasing IL-6 from apical part of airblood barrier. Moreover, TiO2 NPs could not increase the interleukin secretion from basolateral part of airblood barrier [74]. In contrast with Bengalli et al.’s reports, others found an association between TiO2 NPs and microvascular dysfunctions [75]. Nurkiewicz et al. showed that TiO2 NPs inhalation affects microvascular function via reduction of NO bioavailability that was mediated by myeloperoxidase and nicotinamide adenine dinucleotide phosphate oxidase [76]. In contrast, Courtois et al. showed arterial NO-inducedrelaxation was not impaired by TiO2 NPs [77]. In addition, physical interaction of TiO2 NPs with endothelial cell induces the cell leakiness by alteration of VE-cadherin and other junction proteins which is a different molecular mechanism of TiO2 NPs toxicity [78]. The aforementioned evidence suggests that oxidative stress, inflammation, signaling pathways alteration and adhesion molecules expression are the most common mechanisms of TiO2 NPs-induced toxicity in the endothelial cells (see Fig. 2). However, the nitric oxide role and direct nanoparticle-cell membrane interaction should be considered as another possible mechanism.
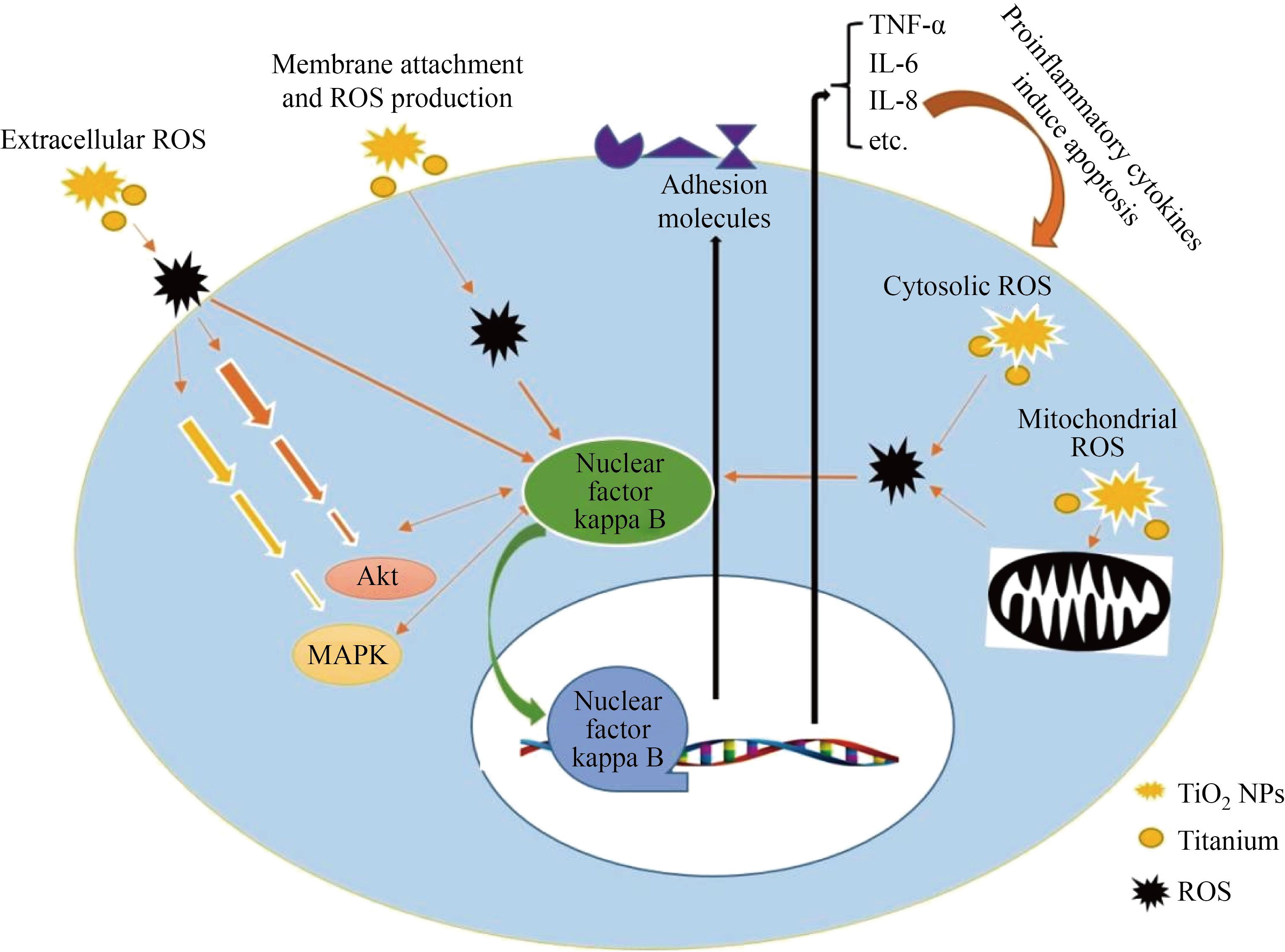
Fig. 2 The toxicity mechanisms of TiO2 NPs on endothelial cells
Hematological parameters and thrombosis
To the best of our knowledge, direct induction of myocardial infraction by TiO2 NPs was not reported but the TiO2 NPs-induced hematological and thrombosis factor alterations that affect the myocardial infraction were reported. TiO2 NPs are absorbed and distributed by the systemic circulation. Therefore, the hematological parameters are imposed to the nanoparticle exposure [79]. Duan et al. showed that intragastric administration of TiO2 NPs (62.5, 125, 250 mg/kg nano-anatase form) reduces and increases the erythrocyte and platelets respectively in dose dependent manner [80]. This study also showed that white blood cells and immunological cells were changed after TiO2 NPs administration. Administration of 50 mg/kg TiO2 NPs leads to hematocrit and platelet count elevation in the adult male wistar rats. Granulocyte, red blood cells, hemoglobin, mean corpuscular volume, mean cell hemoglobin, mean corpuscular hemoglobin concentration, and serum procalcitonin were changed in the high dose nanoparticle treatment (50 mg/kg) [81]. Sang et al. showed that the intragastric administrations of TiO2 NPs at 2.5, 5, and 10 mg/kg for 90 days decrease the blood cells, platelets, hemoglobin, and lymphocyte subsets significantly [82]. However, others showed that intratracheal administration of TiO2 NPs for 28 days did not affect the hematological parameter [37]. An in vitro study on erythrocytes and lymphocytes showed TiO2 NPs reduce the lymphocyte mitochondrial dehydrogenase activity and increase the DNA damage and apoptosis while the membrane integrity was not affected. In addition, TiO2 NPs cause to morphological change and erythrocytes lysis [83]. The oral administration of anatase TiO2 NPs decreases the erythrocyte count and hematocrit, and increases mean corpuscular volume, platelet count, mean platelet volume and white blood cells at higher doses in the rat model. As well as, the abnormal-shaped erythrocytes were observed [84]. Nemmar et al. showed that the intratracheal instillation of TiO2 NPs reduces the number of platelets and increases the monocytes and granulocytes counts [39]. A single dose (1 mg/kg) administration of TiO2 NPs (anatase and rutile) did not alter the leukocyte and platelet counts significantly. Thrombus formation and platelet aggregation were triggered by TiO2 NPs rutile but not anatase form[85]. In contrast, the intravenous injection of TiO2 NPs (140, 300, 645 mg/kg) had no effects on hematological parameter except white blood cells that was increased [86]. TiO2 NPs (intraperitoneal at 20 mg/kg doses every 2 days for 20 days) affect platelets count while other hematological parameters were not affected [87]. Besides the blood cells, serum proteins are involved in the thrombosis including fibrinogens (alpha, beta and gamma units) and complement C9 that interact with TiO2 NPs directly [88]. Hammarström et al. showed that TiO2 NPs (50 ng/mL) trigger kallikrein system. Furthermore, they found that plasma coagulation cascade proteins were adsorbed to the TiO2 NPs that cause to thrombin-antithrombin complex, clot formation and complement system activation [89]. Application of TiO2 NPs nanotube (not nanoparticle) for blood clotting acceleration was proposed as a therapeutic option in the traumatic patients [90]. As well as, there are several studies, those have focusing on the hemolytic effects of TiO2 NPs [91–93]. Taken together, the effects of TiO2 NPs on hematological parameters are undeniable but its possible role in the myocardial infraction at the long term is not well understood.
Vital organ dysfunction
The indirect toxic effects of TiO2 NPs on cardiovascular systems may mediate by other vital organs dysfunction that could be divided into early (acute) and delayed (chronic) effects. The acute effects are the result of ROS and further of pro-inflammatory cytokines production in response of nanoparticle, which causes to a systemic oxidative stress and inflammation [94]. Iavicoli et al. indicated that the inhalation of TiO2 NPs increases the bronchoalveolar fluid cytokines levels, immune cells attraction and a systemic inflammation. A similar scenario could be found in the nervous, skin, liver and gastrointestinal system [22]. Despite there are doubts regarding the validity of in vitro and or in vivo experimental model in the nanotoxicologic assessment [95], we should answer the question whether the oxidative stress and inflammation originating from aforementioned organs are capable to proceed pathological events in the cardiovascular systems? Recently published study shows respiratory exposure causes to persist inflammation and genotoxicity at the higher dose [96].
The CVDs are developed in subjects suffered from respiratory system dysfunction that were referred as pulmonary heart disease [97]. The CVDs are also developed in the chronic kidney and liver diseases [98, 99]. TiO2 NPs induce tissue injury in the several organs including lung, kidney, livers, brain, etc. Therefore, the TiO2 NPs-induced respiratory, renal, liver and nerves system dysfunction might lead to CVDs as a consequence. There are evidence that the long term TiO2 NPs exposure could increase the risk of malignancies, asthma and respiratory dysfunction [100]. Gui et al. found that the chronic TiO2 NPs exposure causes to renal dysfunction that is manifested by the elevation of creatinine and blood urea nitrogen [101]. Cui et al. showed that TiO2 NPs induce the pro-inflammatory pathways activation [102] which is also reported by others [21].
Oxidative stress and inflammation
It is well-documented that oxidative stress and inflammation are the most common molecular mechanisms of TiO2 NPs toxicity, and cardiovascular toxicity is not the exception. The oxidative stress and inflammation have a close interaction and both propagate the subsequent CVDs including atherosclerosis, endothelial dysfunction and lipids metabolism disturbance. Regardless of the exposure route, TiO2 NPs activate the pro-inflammatory signaling pathways specially the NF-κB in the lung, liver, reproductive organs, immune cells and endothelial cells that result in a systemic pro-inflammatory cytokines elevation. Regardless of the origin oxidativeinflammatory factors, the condition could lead to CVDs pathogenesis. The great proportion of articles supports the coincidence of oxidative stress and inflammation due to close crosstalk between them. Meanwhile, there is evidence indicating the nanoparticles could induce endothelial inflammatory responses via various mechanisms other than oxidative stress. In addition, the endothelial cell from different organs show heterogenic response to the nanoparticles toxicity [65]. These observations are supported by Danielsen et al. who showed the TiO2 NPs do not increase the intracellular ROS production but affect the ICAM-1 and VCAM-1 gene expression, and a non-oxidative stress mechanism is considerable for TiO2 NPs [103]. They also showed that the CVDs biomarkers alteration is a function of the composition, size and crystal structure of TiO2 NPs and is independent from particle surface charge. As well as, the anatase form was more toxic than the rutile form. Hassanein et al.’s study on the rat model showed that the elevation of oxidative stress biomarkers and DNA damage were occurred after TiO2 NPs exposure that leads to histopathological changes in the heart [104]. Sha et al. concluded that the effects of TiO2 NPs on heart injury occur with or without presence of oxidative condition. Their results indicated that oxidative stress exacerbates nanoparticle-induced cardiac toxicity [105]. Another study reported that TiO2 NPs increase ROS content of heart (O2-, H2O2, malondialdehyde, and 8-hydroxy-2’-deoxyguanosine) and affect the antioxidant defense component, i.e. superoxide dismutase, glutathione reductase, glutathione-Stransferase, and the levels of antioxidants (including ascorbic acid, glutathione, and thiols), which lead to sparse cardiac muscle fibers, inflammatory response, cell necrosis and cardiac biochemical dysfunction [34]. As well as, other studies confirmed the role of oxidative stress in the TiO2 NPs-cardiac injury [106, 107]. The administration of antioxidant ameliorates the TiO2 NPs-cardiac injury that corroborates the role of oxidative stress in cardiac pathogenesis [108].
Post-exposure oxidative stress and inflammation co-existence were observed in another study, showing that superoxide radical production and Akt, ERK, JNK and p38 signaling pathways activation are induced in the endothelial cells [63]. Using an in vitro experiment on macrophage-like and microvascular cell lines, HanotRoy et al. showed that the DNA damage and heat shock protein 27 and JNK protein alteration were occurred as a stress response [109]. However, opposite results were obtained from a study on human circulating angiogenic cells that indicate the TiO2 NPs (1 to 100 μg/mL) treatment has no effect on oxidative condition and cell death [72].
Intragastric administration of TiO2 NPs at 2.5, 5 or 10 mg/kg for 90 days activates the NF-κB, PKCε and ERK1/2 signaling pathways, and TNF-α, interleukin- 1β, IL-6 and Interferon-α expression were changed via these signaling pathways in the mice model leading to cardiac damage [110]. Husain et al. evaluated the systemic effects of pulmonary deposition to TiO2 NPs on the gene expression profile of the heart, blood and liver that showed the inflammatory genes were expressed in the acute phase response [111]. Daily gastrointestinal administration of TiO2 NPs at 0, 2, 10, 50 mg/kg dosage for 30–90 days increases the serum TNF-α and IL-6 levels. The authors concluded that the inflammatory response is the possible mechanism of cardiovascular toxicity of orally administrated TiO2 NPs [45]. In Hong et al.’s study, the TiO2 NPs decrease ATP production in the hearts and increase expression of NF-κB, interleukin-lβ and TNF-α genes. As well as, expression of anti-inflammatory cytokines including suppressor of cytokine signaling or SOCS1 and SOCS3 was reduced in the cardiac tissue [112]. There are other studies showing that the oxidative stress and inflammation are molecular mechanisms of TiO2 NPs toxicity on cardiovascular system.
Nitrosative stress and vasodilation
Nitric oxide is a short lifetime vasodilator molecule that is altered in CVDs and nitrosative stress. Some experiments found a significant relationship between NO, TiO2 NPs and vasodilation, but other studies rejected the relationship [77]. LeBlanc et al. showed that the TiO2 NPs increase the microvascular ROS contents that impairs the endothelium-dependent vasoactivity [113]. The TiO2 NPs inhalation increased microvascular nitrosative stress significantly that reduces the NO levels in the rat model [76]. Prenatal exposure to TiO2 NPs aerosols (at the 6 day gestational) causes to a significant reduction in the maximal mitochondrial respiration in the left ventricle and impaired the endothelium-dependent dilation [114]. Another study reported that prenatal TiO2 NPs toxicity
on offspring cardiovascular system is mediated via oxidative stress and epigenetic pathways [115].
Heart rate and Blood pressure
Heart rate and blood pressure change could lead to cardiovascular events in the short and long term. Chen et al. showed that the long term administration of TiO2 NPs causes to mild and temporary reduction of heart rate, systolic blood pressure and diastolic blood pressure elevation [45]. Heart rate monitoring in the workers who handle the TiO2 NPs showed that the exposure to particles with a diameter less than 300 nm might affect the heart rate via autonomic nervous system [116]. Kan et al. showed the neurons have a pivotal role in the systemic effects of pulmonary exposure that cause to phosphorylation of p38 mitogen-activated protein kinase and cardiac troponin I in the heart that were medicated via lung-neuron regulated pathway [36]. Single dose (1 mg/kg ) of TiO2 NPs (anatase and rutile) did not alter the mean arterial blood pressure but heart rate was reduced after exposure to rutile form [85]. More recently, a study on animal model showed that the hypertensive rats are much more susceptible to the TiO2 NPs-induced heart injury and failure [117].
The effects of TiO2 NPs on the heart rate, stroke volume index, cardiac index, mean arterial blood
pressure were investigated, which showed all these cardiac function indices are reduced in dose dependent manner [105]. Intragastric TiO2 NPs administration for 9 months affects the cardiac function indices including systolic pressure, maximal rate of pressure increase over time, maximal rate of pressure decrease over time and coronary flow. Furthermore, the left ventricular end-diastolic pressure and heart rate were changed in the mice [112]. The evaluation of TiO2 NPs effects on daphnia magna showed a reduction in the heart rate that disturbs the swimming ability [118]. The mechanisms by which the heart rate and blood pressure are changed were not well-understood. TiO2 NPs may interact with cardiac tissue directly and change the heart rate and blood pressure or via a central nervous system-dependent route. TiO2 NPs could penetrate to the blood brain barrier and cause to neuroinflammation and oxidative damage [119]. On the other hand, the oxidative stress and Neuroinflammation induce blood pressure alteration [120]. Therefore, it is possible that the TiO2 NPs effects on cardiovascular system are mediated via central nervous system-dependent manner [120].
Conclusion and Prospective
Although international organizations consider the TiO2 NPs as the safe particles but current knowledge do not confirm the safety of TiO2 NPs on human being. According to the reviewed documents, the TiO2 NPs toxicity on the cardiovascular system is controversial neither toxicity nor safety of TiO2 NPs is confirmed compellingly but a vigilance on the future studies might be helpful to a straightforward decision. The adult daily consumed is lower than the threshold limit values determined by the National Institute of Occupation Health and Safety [7, 121]. On the other hand, the bioavailability of nanoparticle is concentration dependent and reduced at the higher concentrations [25]. Therefore, an optimum concentration is necessary to reach an effective toxic effects not too low without effect not much higher to aggregate and reduced bioavailability. The questions remaining to be answered are whether the daily exposure amount or worker occupational dealing reaches the CVDs toxic dose or not and whether the occupational exposure limits are high enough to prevent CVDs progression in acute or long terms. In addition, we need a consistence report by considering to the variation in the nanoparticle physicochemical properties, and different experiment settings to conclusion on the TiO2 NPs cardio toxicity.
Conflict of interest
The authors declare that they have no conflict of interest.
References
[1] G. Santulli. Epidemiology of cardiovascular disease in the 21st century: Updated numbers and updated facts. Journal of Cardiovascular Disease Research, 2013, 1: 1.
[2] L. Dai, Y.F. Qi, L.X. Jia, et al. Nanotechnology in Cardiovascular Diseases. Encyclopedia of Nanotechnology. Dordrecht: Springer, 2015: 1–4. https://doi.org/10.1007/978-94-007-6178-0_336-2
[3] Z.L. Han, J.N. Shu, X. Liang, et al. Label-free ratiometric electrochemiluminescence aptasensor based on nanographene oxide wrapped titanium dioxide nanoparticl es with potential-resolved electrochemiluminescence. Analytical Chemistry, 2019, 91: 12260–12267. https://doi.org/10.1021/acs.analchem.9b02318
[4] M.P. Mani, S.K. Jaganathan, A.M. Faudzi, et al. Engineered electrospun polyurethane composite patch combined with Bi-functional components rendering high strength for cardiac tissue engineering. Polymers, 2019, 11: 705. https://doi.org/10.3390/polym11040705
[5] M. P. Sauvant, D. Pepin. Drinking water and cardiovascular disease. Food and Chemical Toxicology, 2002, 40: 1311–1325. https://doi.org/10.1016/S0278- 6915(02)00081-9
[6] B.A. Franklin, R. Brook, C. Arden Pope. Air pollution and cardiovascular disease. Current Problems in Cardiology, 2015, 40: 207–238. https://doi.org/10.1016/j.cpcardiol.2015.01.003
[7] A. Weir, P. Westerhoff, L. Fabricius, et al. Titanium dioxide nanoparticles in food and personal care products. Environmental Science & Technology, 2012, 46: 2242–2250. https://doi.org/10.1021/es204168d
[8] H. Kaminski, M. Beyer, H. Fissan, et al. Measurements of nanoscale TiO2 and Al2O3 in industrial workplace environments - methodology and results. Aerosol and Air Quality Research, 2015, 15: 129–141. https://doi.org/10.4209/aaqr.2014.03.0065
[9] B. Dréno, A. Alexis, B. Chuberre, et al. Safety of titanium dioxide nanoparticles in cosmetics. Journal of the European Academy of Dermatology Venereology, 2019, 33: 34–46. https://doi.org/10.1111/jdv.15943
[10] J. Musial, R. Krakowiak, D.T. Mlynarczyk, et al. Titanium dioxide nanoparticles in food and personal care productswhat do we know about their safety? Nanomaterials (Basel), 2020, 10:1110. doi: 10.3390/nano10061110
[11] A.B. da Silva, M. Miniter, W. Thom, et al. Gastrointestinal absorption and toxicity of nanoparticles and microparticles: Myth, reality and pitfalls explored through titanium dioxide. Current Opinion in Toxicology, 2020, 19: 112–120. https://doi.org/10.1016/j.cotox.2020.02.007
[12] Y.F. Li, J.G. Li, J.L. Yin, et al. Systematic influence induced by 3 nm titanium dioxide following intratracheal instillation of mice. Journal of Nanoscience Nanotechnology, 2010, 10: 8544–8549. https://doi.org/10.1166/jnn.2010.2690
[13] W. Sun, Y.X. Du, J.Q. Chen, et al. Interaction between titanium dioxide nanoparticles and human serum albumin revealed by fluorescence spectroscopy in the absence of photoactivation. Journal of Luminescence, 2009, 129: 778–783. https://doi.org/10.1016/j.jlumin.2009.02.010
[14] R. Tantra, J. Tompkins, P. Quincey. Characterisation of the de-agglomeration effects of bovine serum albumin on nanoparticles in aqueous suspension. Colloids and Surfaces B: Biointerfaces, 2010, 75: 275–281. https://doi.org/10.1016/j.colsurfb.2009.08.049
[15] M. Eydner, D. Schaudien, O. Creutzenberg, et al. Impacts after inhalation of nano- and fine-sized titanium dioxide particles: Morphological changes, translocation within the rat lung, and evaluation of particle deposition using the relative deposition index. Inhalation Toxicology, 2012, 24: 557–569. https://doi.org/10.3109/08958378.2012.697494
[16] W. Souza, S.G. Piperni, P. Laviola, et al. The two faces of titanium dioxide nanoparticles bio-camouflage in 3D bone spheroids. Scientific Reports, 2019, 9: 9309. https://doi.org/10.1038/s41598-019-45797-6
[17] M. Skocaj, M. Filipic, J. Petkovic, et al. Titanium dioxide in our everyday life; is it safe? Radiology and Oncology, 2011, 45: 227–247. https://doi.org/10.2478/v10019-011-0037-0
[18] M. Benčina, A. Iglič, M. Mozetič, et al. Crystallized TiO2 nanosurfaces in biomedical applications. Nanomaterials (Basel), 2020, 10: 1121. https://doi.org/10.3390/nano10061121
[19] 19. T. Kodama. Study on biocompatibility of titanium alloys. Kokubyo Gakkai Zasshi, 1989, 56: 263–288. https://doi.org/10.5357/koubyou.56.263
[20] E. Le Roux. Recent advances on tailor-made titanium catalysts for biopolymer synthesis. Coordination Chemistry Reviews, 2016, 306: 65–85. https://doi.org/10.1016/j.ccr.2015.06.006
[21] H.B. Shi, R. Magaye, V. Castranova, et al. Titanium dioxide nanoparticles: A review of current toxicological data. Particle and Fibre Toxicology, 2013, 10: 15. https://doi.org/10.1186/1743-8977-10-15
[22] I. Iavicoli, V. Leso, A. Bergamaschi. Toxicological effects of titanium dioxide nanoparticles: A review of in vivo studies. Journal of Nanomaterials, 2012, 2012: 5. https://doi.org/10.1155/2012/964381
[23] I. Iavicoli, V. Leso, L. Fontana, et al. Toxicological effects of titanium dioxide nanoparticles: A review of in vitro mammalian studies. European Review for Medical and Pharmacological Sciences, 2011, 15: 481–508.
[24] R.D. Handy, B.J. Shaw. Toxic effects of nanoparticles and nanomaterials: Implications for public health, risk assessment and the public perception of nanotechnology. Health, Risk & Society, 2007, 9: 125–144. https://doi.org/10.1080/13698570701306807
[25] J.X. Wang, Y.B. Fan. Lung injury induced by TiO2 nanoparticles depends on their structural features: Size, shape, crystal phases, and surface coating. International Journal of Molecular Sciences, 2014, 15: 22258–22278. https://doi.org/10.3390/ijms151222258
[26] J.K. Jiang, G. Oberdörster, P. Biswas. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. Journal of Nanoparticle Research, 2009, 11: 77–89. https://doi.org/10.1007/s11051-008-9446-4
[27] M. Shakeel, F. Jabeen, S. Shabbir, et al. Toxicity of nanotitanium dioxide (TiO2-NP) through various routes of exposure: A review. Biological Trace Element Research, 2016, 172: 1–36. https://doi.org/10.1007/s12011-015-0550-x
[28] B. Uttara, A.V. Singh, P. Zamboni, et al. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Current neuropharmacology, 2009, 7: 65–74. https://doi.org/10.2174/157015909787602823
[29] D.A. Pratt, K.A. Tallman, N.A. Porter. Free radical oxidation of polyunsaturated lipids: New mechanistic insights and the development of peroxyl radical clocks. Accounts of chemical Research, 2011, 44: 458–467. https://doi.org/10.1021/ar200024c
[30] J. Hou, L.Y. Wang, C.J. Wang, et al. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. Journal of Environmental Sciences, 2019, 75: 40–53. https://doi.org/10.1016/j.jes.2018.06.010
[31] S.H. Lee, S.K. Jeong, S.K. Ahn. An update of the defensive barrier function of skin. Yonsei Medical Journal, 2006, 47: 293–306. https://doi.org/10.3349/ymj.2006.47.3.293
[32] R.E. Baynes, E. Hodgson. Absorption and distribution of toxicants. A Textbook of Modern Toxicology. Hoboken, NJ, USA: John Wiley & Sons, Inc., 2004: 75–110. https://doi.org/10.1002/0471646776.ch6
[33] E. Huerta-García, M.P. Ramos-Godinez, A. LópezSaavedra, et al. Internalization of titanium dioxide nanoparticles is mediat ed by actin-dependent reorganization and clathrin- and dynamin-mediated endocytosis in H9c2 rat cardiomyoblasts. Chemical Research in Toxicology, 2019, 32: 578–588. https://doi.org/10.1021/acs.chemrestox.8b00284
[34] L. Sheng, X.C. Wang, X.Z. Sang, et al. Cardiac oxidative damage in mice following exposure to nanoparticulate titanium dioxide. Journal of Biomedical Materials Research Part A, 2013, 101: 3238–3246. https://doi.org/10.1002/jbm.a.34634
[35] M. Savi, S. Rossi, L. Bocchi, et al. Titanium dioxide nanoparticles promote arrhythmias via a direct interaction with rat cardiac tissue. Particle and Fibre Toxicology, 2014, 11: 63. https://doi.org/10.1186/s12989-014-0063-3
[36] H. Kan, Z.X. Wu, S.H. Young, et al. Pulmonary exposure of rats to ultrafine titanium dioxide enhances cardiac protein phosphorylation and substance P synthesis in nodose Ganglia. Nanotoxicology, 2012, 6: 736–745. https://doi.org/10.3109/17435390.2011.611915
[37] X.H. Chang, Y.X. Xie, J.R. Wu, et al. Toxicological characteristics of titanium dioxide nanoparticle in rats. Journal of Nanoscience and Nanotechnology, 2015, 15: 1135–1142. https://doi.org/10.1166/jnn.2015.8998
[38] J.X. Wang, G.Q. Zhou, C.Y. Chen, et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration.Toxicology Letters, 2007, 168: 176–185. https://doi.org/10.1016/j.toxlet.2006.12.001
[39] A. Nemmar, K. Melghit, B.H. Ali. The acute proinflammatory and prothrombotic effects of pulmonary exposure to rutile TiO2 nanorods in rats. Experimental Biology and Medicine, 2008, 233: 610–619. https://doi.org/10.3181/0706-rm-165
[40] F.S. Hong, L. Wang, X.H. Yu, et al. Toxicological effect of TiO2 nanoparticle-induced myocarditis in mice. Nanoscale Research Letters, 2015, 10: 326. https://doi.org/10.1186/s11671-015-1029-6
[41] H.T. Liu, L.L. Ma, J.F. Zhao, et al. Biochemical toxicity of nano-anatase TiO2 particles in mice. Biological Trace Element Research, 2009, 129: 170–180. https://doi.org/10.1007/s12011-008-8285-6
[42] J.Y. Chen, X. Dong, J. Zhao, et al. In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. Journal of Applied Toxicology, 2009, 29: 330–337. https://doi.org/10.1002/jat.1414
[43] D. Elgrabli, R. Beaudouin, N. Jbilou, et al. Biodistribution and clearance of TiO2 nanoparticles in rats after intravenous injection. PLoS One, 2015, 10: e0124490. https://doi.org/10.1371/journal.pone.0124490
[44] Y. Wang, Z.J. Chen, T. Ba, et al. Susceptibility of young and adult rats to the oral toxicity of titanium dioxide nanoparticles. Small, 2013, 9: 1742–1752. https://doi.org/10.1002/smll.201201185
[45] Z.J. Chen, Y. Wang, L. Zhuo, et al. Effect of titanium dioxide nanoparticles on the cardiovascular system after oral administration. Toxicology Letters, 2015, 239: 123–130. https://doi.org/10.1016/j.toxlet.2015.09.013
[46] X.H. Yu, X.Y. Zhao, Y.G. Ze, et al. Changes of serum parameters of TiO2 nanoparticle-induced atherosclerosis in mice. Journal of Hazardous Materials, 2014, 280: 364–371. https://doi.org/10.1016/j.jhazmat.2014.08.015
[47] T. Chen, J.Q. Hu, C.Y. Chen, et al. Cardiovascular effects of pulmonary exposure to titanium dioxide nanoparticles in ApoE knockout mice. Journal of Nanoscience and Nanotechnology, 2013, 13: 3214–3222. https://doi.org/10.1166/jnn.2013.7147
[48] L. Mikkelsen, M. Sheykhzade, K.A. Jensen, et al. Modest effect on plaque progression and vasodilatory function in atherosclerosis-prone mice exposed to nanosized TiO(2). Particle and Fibre Toxicology, 2011, 8: 32. https://doi.org/10.1186/1743-8977-8-32
[49] J.Q. Hu, C.Y. Chen, R. Bai, et al. Effect of nanoTiO(2) intratracheal instillation on lipid metabolism of AopE gene-knockout mice. Chinese Journal of Preventive Medicine, 2010, 44: 780–784. https://doi.org/10.3760/CMA.J.ISSN.0253-9624.2010.09.004
[50] M. Tang, T. Zhang, Y.Y. Xue, et al. Metabonomic studies of biochemical changes in the serum of rats by intratracheally instilled TiO2 nanoparticles. Journal of Nanoscience and Nanotechnology, 2011, 11: 3065–3074. https://doi.org/10.1166/jnn.2011.3604
[51] H.L. Hu, Q. Guo, C.L. Wang, et al. Titanium dioxide nanoparticles increase plasma glucose via reactive oxygen species-induced insulin resistance in mice. Journal of Applied Toxicology, 2015, 35: 1122–1132. https://doi.org/10.1002/jat.3150
[52] Z.J. Chen, D. Zhou, Y. Wang, et al. Combined effect of titanium dioxide nanoparticles and glucose on the cardiovascular system in young rats after oral administration. Journal of Appllied Toxicology, 2019, 39: 590–602. https://doi.org/10.1002/jat.3750
[53] Y. Suzuki, S. Tada-Oikawa, G. Ichihara, et al. Zinc oxide nanoparticles induce migration and adhesion of monocytes to endothelial cells and accelerate foam cell formation. Toxicology and Applied Pharmacology, 2014, 278: 16–25. https://doi.org/10.1016/j.taap.2014.04.010
[54] M. Giovanni, J. Yue, L. Zhang, et al. Pro-inflammatory responses of RAW264.7 macrophages when treated with ultralow concentrations of silver, titanium dioxide, and zinc oxide nanoparticles. Journal of Hazardous Materials, 2015, 297: 146–152. https://doi.org/10.1016/j.jhazmat.2015.04.081
[55] S. Triboulet, C. Aude-Garcia, L. Armand, et al. Comparative proteomic analysis of the molecular responses of mouse macrophages to titanium dioxide and copper oxide nanoparticles unravels some toxic mechanisms for copper oxide nanoparticles in macrophages. PLoS One, 2015, 10: e0124496. https://doi.org/10.1371/journal.pone.0124496
[56] R. Liu, X.Y. Zhang, Y.P. Pu, et al. Small-sized titanium dioxide nanoparticles mediate immune toxicity in rat pulmonary alveolar macrophages in vivo. Journal of Nanoscience and Nanotechnology, 2010, 10: 5161–5169. https://doi.org/10.1166/jnn.2010.2420
[57] W.C. Xu, X. Dong, J.L. Ding, et al. Nanotubular TiO2 regulates macrophage M2 polarization and increases macrophage secretion of VEGF to accelerate endothelialization via the ERK1/2 and PI3K/AKT pathways. International Journal of Nanomedicine, 2019, 14: 441–455. https://doi.org/10.2147/ijn.s188439
[58] Y. Hou, M. Lai, X. Chen, et al. Effects of mesoporous SiO2, Fe3 O4, and TiO2 nanoparticles on the biological functions of endothelial cells in vitro. Journal of Biomedical Materials Research Part A, 2014, 102: 1726–1736. https://doi.org/10.1002/jbm.a.34839
[59] Z. Gholinejad, A. Ghasemian, Y. Tutar, et al. N-acetyl cysteine and metal nanoparticles internalization: A critical methodological aspect. Journal of Bionanoscience, 2018, 12: 700–704. https://doi.org/10.1166/jbns.2018.1587
[60] E. Huerta-García, J.A. Pérez-Arizti, S.G. MárquezRamírez, et al. Titanium dioxide nanoparticles induce strong oxidative stress and mitochondrial damage in glial cells. Free Radical Biology and Medicine, 2014, 73: 84–94. https://doi.org/10.1016/j.freeradbiomed.2014.04.026
[61] A. Montiel-Dávalos, J.L. Ventura-Gallegos, E. AlfaroMoreno, et al. TiO2 nanoparticles induce dysfunction and activation of human endothelial cells. Chemical Research in Toxicology, 2012, 25: 920–930. https://doi.org/10.1021/ tx200551u
[62] M.D.E.L.P. Ramos-Godínez, B.E. González-Gómez, A. Montiel-Dávalos, et al. TiO2 nanoparticles induce endothelial cell activation in a pneumocyte-endothelial co-culture model. Toxicology in Vitro Int. J. Publ. Assoc. BIBRA, 2013, 27: 774–781. https://doi.org/10.1016/j.tiv.2012.12.010
[63] S.G. Han, B. Newsome, B. Hennig. Titanium dioxide nanoparticles increase inflammatory responses in vascular endothelial cells. Toxicology, 2013, 306: 1–8. https://doi.org/10.1016/j.tox.2013.01.014
[64] S. Guney-Ayra, et al. Induction of oxidative stress, lysosome activation and autophagy by nanoparticles in human brain-derived endothelial cells. Biochemical Journal, 2012, 441: 813–821. https://doi.org/10.1042/bj20111252
[65] R. Alinovi, M. Goldoni, S. Pinelli, et al. Oxidative and pro-inflammatory effects of cobalt and titanium oxide nanoparticles on aortic and venous endothelial cells. Toxicology in Vitro, 2015, 29: 426–437. https://doi.org/10.1016/j.tiv.2014.12.007
[66] Z. Gholinejad, M.H. Khadem Ansari, Y. Rasmi. Titanium dioxide nanoparticles induce endothelial cell apoptosis via cell membrane oxidative damage and p38, PI3K/Akt, NF-κB signaling pathways modulation. Journal of Trace Elements in Medicine and Biology, 2019, 54: 27–35. https://doi.org/10.1016/j.jtemb.2019.03.008
[67] Q.A. Hathaway, A.J. Durr, D.L. Shepherd, et al. miRNA- 378a as a key regulator of cardiovascular health following engineered nanomaterial inhalation exposure. Nanotoxicology, 2019, 13: 644–663. https://doi.org/10.1080/17435390.2019.1570372
[68] Q. Q. Yan, L. Yang, J. Zhao, et al. Comparative experiment on nanoparticle-induced toxicity in human vascular endothelial cells. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi, Chinese Journal of Industrial Hygiene and Occupational Diseases 2012, 30: 820-824.
[69] L. Peng, A.J. Barczak, R.A. Barbeau, et al. Whole genome expression analysis reveals differential effects of TiO2 nanotubes on vascular cells. Nano Letters, 2010, 10: 143–148. https://doi.org/10.1021/nl903043z
[70] Y.X. Gu, S.S. Cheng, G. Chen, et al. The effects of endoplasmic reticulum stress inducer thapsigargin on the toxicity of ZnO or TiO2 nanoparticles to human endothelial cells. Toxicology Mechanisms and Methods, 2017, 27: 191–200. https://doi.org/10.1080/15376516.2016.1273429
[71] N. Bayat, V.R. Lopes, J. Schölermann, et al. Vascular toxicity of ultra-small TiO2 nanoparticles and single walled carbon nanotubes in vitro and in vivo. Biomaterials, 2015, 63: 1–13. https://doi.org/10.1016/j.biomaterials.2015.05.044
[72] V. Spigoni, M. Cito, R. Alinovi, et al. Effects of TiO2 and Co3O4 nanoparticles on circulating angiogenic cells. PLoS One, 2015, 10: e0119310. https://doi.org/10.1371/journal. pone.0119310
[73] S. Smulders, K. Luyts, G. Brabants, et al. Toxicity of nanoparticles embedded in paints compared to pristine nanoparticles, in vitro study. Toxicology Letters, 2015, 232: 333–339. https://doi.org/10.1016/j.toxlet.2014.11.030
[74] R. Bengalli, P. Mantecca, M. Camatini, et al. Effect of nanoparticles and environmental particles on a cocultures model of the air-blood barrier. Biomed Res. Int., 2013, 2013: 801214. https://doi.org/10.1155/2013/801214
[75] T.R. Nurkiewicz, D.W. Porter, A.F. Hubbs, et al. Pulmonary particulate matter and systemic microvascular dysfunction. Research Report (Health Effects Institute), 2011: 3–48.
[76] T.R. Nurkiewicz, D.W. Porter, A.F. Hubbs, et al. Pulmonary nanoparticle exposure disrupts systemic microvascular nitric oxide signaling. Toxicological Science, 2009, 110: 191–203. https://doi.org/10.1093/toxsci/kfp051
[77] A. Courtois, P. Andujar, Y. Ladeiro, et al. Impairment of NO-dependent relaxation in intralobar pulmonary arteries: Comparison of urban particulate matter and manufactured nanoparticles. Environmental Health Perspectives, 2008, 116: 1294–1299. https://doi.org/10.1289/ehp.11021
[78] M.I. Setyawati, C.Y. Tay, S.L. Chia, et al. Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE-cadherin. Nature Communications, 2013, 4: 1673. https://doi.org/10.1038/ncomms2655
[79] W.I. Hagens, A.G. Oomen, W.H. de Jong, et al. What do we (need to) know about the kinetic properties of nanoparticles in the body? Regulatory Toxicology and Pharmacology, 2007, 49: 217–229. https://doi.org/10.1016/j.yrtph.2007.07.006
[80] Y.M. Duan, J. Liu, L.L. Ma, et al. Toxicological characteristics of nanoparticulate anatase titanium dioxide in mice. Biomaterials, 2010, 31: 894–899. https://doi.org/10.1016/j.biomaterials.2009.10.003
[81] D. Vasantharaja, V. Ramalingam, G. Reddy. Titanium dioxide nanoparticles induced alteration in haematological indices of adult male wistar rats. Journal of Academia and Industrial Research, 2015.3: 632–635.
[82] X.Z. Sang, L. Zheng, Q. Q. Sun, et al. The chronic spleen injury of mice following long-term exposure to titanium dioxide nanoparticles. Journal of Biomedical Materials Research Part A, 2012, 100A: 894–902. https://doi.org/10.1002/jbm.a.34024
[83] M. Ghosh, A. Chakraborty, A. Mukherjee. Cytotoxic, genotoxic and the hemolytic effect of titanium dioxide (TiO2) nanoparticles on human erythrocyte and lymphocyte cells in vitro. Journal of Applied Toxicology, 2013, 33: 1097–1110. https://doi.org/10.1002/jat.2863
[84] I. Grissa, J. Elghoul, L. Ezzi, et al. Anemia and genotoxicity induced by sub-chronic intragastric treatment of rats with titanium dioxide nanoparticles. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 2015, 794: 25–31. https://doi.org/10.1016/j.mrgentox.2015.09.005
[85] N. Haberl, S. Hirn, M. Holzer, et al. Effects of acute systemic administration of TiO2, ZnO, SiO2, and Ag nanoparticles on hemodynamics, hemostasis and leukocyte recruitment. Nanotoxicology, 2015, 9: 963–971. https://doi.org/10.3109/17435390.2014.992815
[86] J.Y. Xu, H.B. Shi, M. Ruth, et al. Acute toxicity of intravenously administered titanium dioxide nanoparticles in mice. PLoS One, 2013, 8: e70618. https://doi.org/10.1371/journal.pone.0070618
[87] N.R. Ben Younes, S. Amara, I. Mrad, et al. Subacute toxicity of titanium dioxide (TiO2) nanoparticles in male rats: Emotional behavior and pathophysiological examination. Environmental Science and Pollution Research, 2015, 22: 8728–8737. https://doi.org/10.1007/s11356-014-4002-5
[88] Z.J. Deng, G. Mortimer, T. Schiller, et al. Differential plasma protein binding to metal oxide nanoparticles. Nanotechnology, 2009, 20: 455101. https://doi.org/10.1088/0957-4484/20/45/455101
[89] B. Ekstrand-Hammarström, J. Hong, P. Davoodpour, et al. TiO2 nanoparticles tested in a novel screening whole human blood model of toxicity trigger adverse activation of the kallikrein system at low concentrations. Biomaterials, 2015, 51: 58–68. https://doi.org/10.1016/j.biomaterials.2015.01.031
[90] S.C. Roy, M. Paulose, C.A. Grimes. The effect of TiO2 nanotubes in the enhancement of blood clotting for the control of hemorrhage. Biomaterials, 2007, 28: 4667–4672. https://doi.org/10.1016/j.biomaterials.2007.07.045
[91] B.M. Rothen-Rutishauser, S. Schürch, B. Haenni, et al. Interaction of fine particles and nanoparticles with red blood cells visualized with advanced microscopic techniques. Environmental Science & Technology, 2006, 40: 4353–4359. https://doi.org/10.1021/es0522635
[92] Y. Aisaka, R. Kawaguchi, S. Watanabe, et al. Hemolysis caused by titanium dioxide particles. Inhalation Toxicology, 2008, 20: 891–893. https://doi.org/10.1080/08958370802304123
[93] N.N. Zhou, X.B. Lin, D.G. Liu, et al. Efficacy and toxicity of trastuzumab combined with docetaxel for Her-2/neu overexpressing metastatic breast cancer. Chinese Journal of Cancer, 2008, 27: 947–950.
[94] M. Entezari, F. Ghanbary. Toxicity of Manganese titanate on rat vital organ mitochondria. Iranian Journal of Pharmaceutical Research, 2019, 18: 713–719. https://doi.org/10.22037/ijpr.2019.1100639
[95] M.J.D. Clift, P. Gehr, B. Rothen-Rutishauser. Nanotoxicology: A perspective and discussion of whether or not in vitro testing is a valid alternative. Archives of Toxicology, 2011, 85: 723–731. https://doi.org/10.1007/s00204-010-0560-6
[96] C. Relier, M. Dubreuil, O. Lozano Garcia, et al. Study of TiO2 P25 nanoparticles genotoxicity on lung, blood, and liver cells in lung overload and non-overload conditions after repeated respiratory exposure in rats. Toxicological Sciences, 2017, 156: 527–537. https://doi.org/10.1093/toxsci/kfx006
[97] P.R. Forfia, A. Vaidya, S.E. Wiegers. Pulmonary heart disease: The heart-lung interaction and its impact on patient phenotypes. Pulmonary Circulation, 2013, 3: 5–19. https://doi.org/10.4103/2045-8932.109910
[98] J. Wright, A. Hutchison. Cardiovascular disease in patients with chronic kidney disease. Vascular Health and Risk Management, 2009, 5: 713–722. https://doi.org/10.2147/vhrm.s6206
[99] G. Fede, G. Privitera, T. Tomaselli, et al. Cardiovascular dysfunction in patients with liver cirrhosis. Annals of Gastroenterology, 2015, 28: 31–40.
[100] Y. Morimoto, H. Izumi, E. Kuroda. Significance of persistent inflammation in respiratory disorders induced by nanoparticles. Journal of Immunology Research, 2014. https://doi.org/10.1155/2014/962871
[101] S.X. Gui, B.Y. Li, X.Y. Zhao, et al. Renal injury and Nrf2 modulation in mouse kidney following chronic exposure to TiO2 nanoparticles. Journal of Agricultural and Food Chemistry, 2013, 61: 8959–8968. https://doi.org/10.1021/jf402387e
[102] Y.L. Cui, H.T. Liu, M. Zhou, et al. Signaling pathway of inflammatory responses in the mouse liver caused by TiO2 nanoparticles. Journal of Biomedical Materials Research Part A, 2011, 96: 221–229. https://doi.org/10.1002/jbm. a.32976
[103] P.H. Danielsen, Y. Cao, M. Roursgaard, et al. Endothelial cell activation, oxidative stress and inflammation induced by a panel of metal-based nanomaterials. Nanotoxicology, 2015, 9: 813–824. https://doi.org/10.3109/17435390.2014. 980449
[104] K.M.A. Hassanein, Y.O. El-Amir. Protective effects of thymoquinone and avenanthramides on titanium dioxide nanoparticles induced toxicity in Sprague-Dawley rats. Pathology-Research and Practice, 2017, 213: 13–22. https://doi.org/10.1016/j.prp.2016.08.002
[105] B.Y. Sha, W. Gao, S.Q. Wang, et al. Nano-titanium dioxide induced cardiac injury in rat under oxidative stress. Food and Chemical Toxicology, 2013, 58: 280–288. https://doi.org/10.1016/j.fct.2013.04.050
[106] C.E. Nichols, D.L. Shepherd, Q.A. Hathaway, et al. Reactive oxygen species damage drives cardiac and mitochondrial dysfunction following acute nano-titanium dioxide inhalation exposure. Nanotoxicology, 2018, 12: 32–48. https://doi.org/10.1080/17435390.2017.1416202
[107] E. Huerta-García, I. Zepeda-Quiroz, H. Sánchez-Barrera, et al. Internalization of titanium dioxide nanoparticles is cytotoxic for H9c2 rat cardiomyoblasts. Molecules, 2018, 23: 1955. https://doi.org/10.3390/molecules23081955
[108] E.A. El-Din, H.E. Mostafa, M.A. Samak, et al. Could curcumin ameliorate titanium dioxide nanoparticles effect on the heart? A histopathological, immunohistochemical, and genotoxic study. Environmental Science and Pollution Research, 2019, 26: 21556–21564. https://doi.org/10.1007/s11356-019-05433-2
[109] M. Hanot-Roy, E. Tubeuf, A. Guilbert, et al. Oxidative stress pathways involved in cytotoxicity and genotoxicity of titanium dioxide (TiO2) nanoparticles on cells constitutive of alveolo-capillary barrier in vitro. Toxicology in Vitro, 2016, 33: 125–135. https://doi.org/10.1016/j.tiv.2016.01.013
[110] X.H. Yu, F.S. Hong, Y.Q. Zhang. Cardiac inflammation involving in PKCε or ERK1/2-activated NF-κB signalling pathway in mice following exposure to titanium dioxide nanoparticles. Journal of Hazardous Materials, 2016, 313: 68–77. https://doi.org/10.1016/j.jhazmat.2016.03.088
[111] M. Husain, D.M. Wu, A.T. Saber, et al. Intratracheally instilled titanium dioxide nanoparticles translocate to heart and liver and activate complement cascade in the heart of C57BL/6 mice. Nanotoxicology, 2015, 9: 1013–1022. https://doi.org/10.3109/17435390.2014.996192
[112] F.S. Hong, N. Wu, X.Y. Zhao, et al. Titanium dioxide nanoparticle-induced dysfunction of cardiac hemodynamics is involved in cardiac inflammation in mice. Journal of Biomedical Materials Research Part A, 2016, 104: 2917–2927. https://doi.org/10.1002/jbm.a.35831
[113] A.J. LeBlanc, A.M. Moseley, B.T. Chen, et al. Nanoparticle inhalation impairs coronary microvascular reactivity via a local reactive oxygen species-dependent mechanism. Cardiovascular Toxicology, 2010, 10: 27–36. https://doi.org/10.1007/s12012-009-9060-4
[114] P.A. Stapleton, C.E. Nichols, J.H. Yi, et al. Microvascular and mitochondrial dysfunction in the female F1 generation after gestational TiO2 nanoparticle exposure. Nanotoxicology, 2015, 9: 941–951. https://doi.org/10.3109/17435390.2014.984251
[115] A. Kunovac, Q.A. Hathaway, M.V. Pinti, et al. ROS promote epigenetic remodeling and cardiac dysfunction in offspring following maternal engineered nanomaterial (ENM) exposure. Particle and Fibre Toxicology, 2019, 16: 24. https://doi.org/10.1186/s12989-019-0310-8
[116] S. Ichihara, W.H. Li, S. Omura, et al. Exposure assessment and heart rate variability monitoring in workers handling titanium dioxide particles: A pilot study. Journal of Nanoparticle Research, 2016, 18: 52. https://doi.org/10.1007/s11051-016-3340-2
[117] S. Rossi, M. Savi, M. Mazzola, et al. Subchronic exposure to titanium dioxide nanoparticles modifies cardiac structure and performance in spontaneously hypertensive rats. Particle and Fibre Toxicology, 2019, 16: 25. https://doi.org/10.1186/s12989-019-0311-7
[118] W. Chung, J. Song, J. Lee. The evaluation of titanium dioxide nanoparticle effects on cardiac and swimming performance of Daphnia magna. International Journal of Applied Environmental Sciences, 2016. 11(6): 1375–1385.
[119] B. Song, J. Liu, X.L. Feng, et al. A review on potential neurotoxicity of titanium dioxide nanoparticles. Nanoscale Research Letters, 2015, 10: 1042. https://doi.org/10.1186/s11671-015-1042-9
[120] P.J. Winklewski, M. Radkowski, M. WszedybylWinklewska, et al. Brain inflammation and hypertension: The chicken or the egg? Journal of Neuroinflammation 2015, 12: 85. https://doi.org/10.1186/s12974-015-0306-8
[121] I.A. Mudunkotuwa, T.R. Anthony, V.H. Grassian, et al. Accurate quantification of TiO2 nanoparticles collected on air filters using a microwave-assisted acid digestion method. Journal of Occupational and Environmental Hygiene, 2016, 13: 30–39. https://doi.org/10.1080/15459624.2015.1072278
Copyright© Shiva Mehran, Soroush Ghodratizadeh, Ali ZolfiGol, Hamed Charkhian, Mojtaba Ranjbari, Vahed ebrahimi and Zafar Gholinejad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.