Green Synthesis, Characterization and Antiepileptic Activity of Herbal Nanoparticles of Mimusops Elengi in Mice
Jayaraman Rajangam1*, Pushpalatha Sampathi2, Narahari Narayan Palei1, Anna Balaji2, Shyam Sundar A.3, Bibhash Chandra Mohanta4, Vasanth Raj Palanimuthu5
1Amity Institute of Pharmacy, Amity University, Lucknow, India
2Department of Pharmacology, Sree Vidyanikethan College of Pharmacy, Tirupati, India
3College of Pharmacy and Nursing, University of Nizwa, Nizwa, Oman
4College of Pharmacy, Teerthanker Mahaveer University, Uttar Pradesh, India
5Department of Pharmaceutical Biotechnology, JSS College of Pharmacy Ooty, Tamil Nadu, India
*Corresponding author. E-mail: jayaraam81@gmail.com
Received: Nov. 23, 2021; Revised: Mar. 01, 2022; Accepted: Dec. 29, 2022; Published: Dec. 31, 2022
Citation: J. Rajangam, P. Sampathi, N. Palei, et al. Green synthesis, characterization and antiepileptic activity of herbal nanoparticles of Mimusops elengi in mice. Nano Biomedicine and Engineering, 2022, 14(4): 295–307.
DOI: 10.5101/nbe.v14i4.p295-307
Abstract
The aim of the study was to green synthesis and evaluate the antiepileptic activity of herbal nanoparticles of Mimusops elengi (ME) on electrochemical convulsion models in mice. ME herbal nanoparticles (MEHNPs) were synthesized and characterized. The particles size and zeta potential of MEHNPs were found to be 24 nm and 29.5 mV respectively. FTIR study revealed that minor shifts of peaks may be due to capping, reduction and stabilization of MEHNPs. Electro convulsions (ECs) were induced using electro-convulsiometer and ear-clip electrodes with alternating current of 45 mA for 0.2 s stimulation. PTZ was administered intraperitoneally to mice at a dose of 105 mg/kg, which was the CD97 (97% convulsive dose for the clonic phase) for inducing chemo-convulsions. The ability of methanol extract of ME bark (MEME-B) and ME herbal nanoparticles (MEHNPs) to reduce the hind limb extension (HLE) in EC and clonic-type (CT) convulsions in the PTZ model was used to establish antiepileptic parameters. HLE and CC was reduced significantly (P < 0.05) whereas neurochemical analyses show a significant rise in Acetylcholinesterase (AChE) and Gamma Butyric acid (GABA) levels. Antioxidant studies revealed that both MEME and MEHNPs significantly increased antioxidant enzymes and scavenged free radicals (P < 0.05), whereas histological findings revealed the protective effects. In both models, MEME-B and MEHNPs exhibited significant anti-epileptic properties, which could be attributed to suppressing excitatory neurohumoral transmission and enhanced inhibitory neurotransmission, as well as a significant increase in antioxidant status. MEHNPs, on the other hand, have outperformed MEME-B in terms of reducing oxidative stress and altering neurochemicals such as AChE and GABA.
Keywords: Green synthesis; Mimusops elengi; Herbal nanoparticles; Electro convulsions; Chemo
convulsions; Acetylcholinesterase; Antioxidants
Introduction
Epilepsy is one of the neuronal sicknesses of the brain stemming from the disruption of brain signaling process. It is mainly characterized by an enduring predisposition to generate epileptic seizures, which can have neurobiological, cognitive, psychological, and social consequences, putting the individual at risk of physical harm and frequently interfering with quality of life at any age [1, 2]. Epilepsy affects almost all age groups and is estimated to affect 50 million people worldwide, with 75% of patients living in economically deprived countries with little or no access to adequate care options, ranking it second only to stroke as one of the most prevalent severe neurological disorders [3, 4].
The treatment strategy for people with epilepsy, on the other hand, is the continuous and regular use of anticonvulsants [5]. However, current medicinal agents and treatment methods, either as monotherapy or in combination therapy, are inadequate for approximately one-third of sufferers worldwide, and 30% of patients with epilepsy do not have sufficient seizure control regardless of available drugs or treatment choices [6–8]. Furthermore, according to the data, thousands of fatalities occur in the United Kingdom each year. Because of epilepsy, and most patients are associated with seizures, 42% of deaths were potentially avoidable [9]. Hence, improved treatment plan options or newer antiepileptic agents as monotherapy or as an add-on medication for epilepsy and associated comorbid conditions are urgently needed. By considering the above fact, considerably more attention is now being paid to the plant's research as an effective cure for most of the disease. Furthermore, according to data, more than 70% of medicines were isolated from plants as primary and secondary metabolites, converted into active ingredients, and used to treat various illnesses, including cardiovascular and central nervous system disorders.
ME is a member of the Sapotaceae family, which is widely distributed throughout the world, especially in Asian countries such as India, Pakistan, and Bangladesh. This is also called Bakul with many active components such as flavonoids, tannins, triterpenes, saponins [10, 11]. Plant extracts of ME parts have historically been claimed and published for antiulcer, antibacterial, anthelmintic, and cardiotonic activities using experimental animal models. Phytochemicals such as beta amyrin, betulinic acid, lupeol, betasitosterol, ursolic acid, and quercetin have been isolated from the bark of ME [12, 13]. Ursolic acid is classified as a triterpene acid with various biological effects, including strong antioxidant properties [14].
On the other hand, emerging formulations such as polymeric herbal nanoparticles, liposomes, proliposomes, solid lipid nanoparticles, and nanoemulsions, have recently attracted a lot of interest
in herbal research area. Furthermore, in phytoformulation research, phytotherapeutics also have various advantages such as higher solubility with improved bioavailability, improved stability, improved tissue macrophage distribution along with non toxic in nature and so on. As a result, nano-sized drug delivery systems for herbal treatments may have a promising future in terms of improving activity and overcoming issues connected with plant medications [15, 16]. In drug delivery, many forms and methods for successful delivery of medicaments to target tissues have been identified in available reports. In the current scenario, nanotechnology drug design provides a more promising drug delivery solution for various treatments. Furthermore, multiple nanostructure drug delivery carriers have been used and published as successful CNS delivery systems in recent days [17, 18]. In this approach, active medicaments are usually embedded into or coated onto nanocarriers (size from 1 to 1000 nm) to interact with the cell organelles to produce any biological responses. Hence, nanomedicine-based AEDs show greater attention because of their ability to cross the BBB with improved selectivity and potential for effective drug delivery approaches [19]. In view of the above, the nanoparticles from ME were prepared and evaluated for their potential antiepileptic activity on convulsion models in mice. To our knowledge, this is the first time that herbal nanoparticle formulation from ME bark has been tested in mice using electro and chemo convulsion models.
Experiment
Drugs, chemicals, and plant materials
Phenytoin, Diazepam, and Pentylenetetrazole were procured from Yarrow Chem Products, Mumbai, India. Silver nitrate was purchased from Hi-Media Chemicals, Mumbai, India. Methanol with purity of 99.7%, acetonitrile with a purity of 99.8% and distilled water was used as a mobile phase and procured from local chemical supplier (Bros chemicals, Tirupati, Andhra Pradesh, India). The reference compound ursolic acid with a purity of 90% was procured from Sigma-Aldrich, USA. All the chemicals and solvents used in the study were of analytical grade. ME bark was collected from Sree Vidyanikethan College campus, identified and authenticated by Dr. K. Madhavachetty, Professor, Faculty of Botony, Sri Venkateswara University, Tirupati-517 502 Andhra Pradesh. A voucher specimen (Reference No: #45 Dated 07/11/2018) was deposited for further reference. The collected and authenticated bark was then washed with standard water to extract dust and residual particles, dry, powdered, and passed through the BSS mesh No. 85, and the resulting powder was held in an airtight container for further assessment and quantification.
Preparation and characterization of ME bark
ME bark was collected, chopped, shade-dried, and coarsely powdered. The powder (100 g) was passed through a 40-mesh sieve and extracted with methanol using the soxhlet apparatus. The solvent was then removed and concentrated to dryness in a rotary vacuum evaporator at 40 ℃ under reduced pressure [20].
Phytochemical studies
The phytochemical screening of ME barks extract was carried out by using the standard methods and procedures for terpenoids, alkaloids, flavonoids, tannins, steroids, and saponins [21, 22].
FT-IR spectrum of extract
The FT-IR spectra were captured using Agilent Cary 630 IR systems. A weighed volume of dried methanol extract of ME (MEME-B) was carefully and directly loaded into the instrument’s sample holder. Data were collected over a spectral range of 4000 to 800 cm–1. Agilent-Respro software was used to evaluate all of the collected data.
Quantification of ursolic acid in extract by HPLC analysis
A Shimadzu LC 20AT isocratic pump was used for HPLC analysis. As a stationary phase, a reversephase Phenomenex RP-C18 (150 mm×4.60 mm, 5 mm particle size) was used. A UV detector was used for detection. Data were collected using Spinchrom software. The injection volume was optimized as 10 µL. The mobile process was composed of water and acetonitrile in an 80:20 volume ratio. The wavelength of detection was set to 210 nm [23]. Ursolic acid was combined with water to make a stock solution with a concentration of 1000 g/mL. Other dilutions have been made for the preparation of regular solutions from the stock solution. The extract of 1 g of bark in 10 mL methanol for sample solution was filtered through a millipore membrane filter (0.45 μm).
Preparation and characterization of MEHNPs
For the biosynthesis, 100 mL of silver nitrate (1 mmol/L) was prepared. In a 250 mL erlenmeyer flask, 10 mL ME extract was added to 90 mmol/L of 1 mmol/L silver nitrate aqueous solution, and the mixture was stirred for 2 h at 60–70 ℃. MEHNPs were formed as soon as the reduction of silver ions which change the reaction color from light yellow to brown [24].
Particle size and zeta potential
The average particle size and zeta potential were measured using a dynamic light scattering technique (Horiba SZ100, Japan). The average particle size and zeta potential were determined by diluting dispersion 100 times with deionized water and taking measurements at a 90° angle [25].
Transmission electron microscopy (TEM) and EDAX studies
The morphology of the synthesized MEHNP dispersion was determined using TEM (JEOL JEM- 2100, JAPAN) operated at an accelerating voltage of 15000 V. One drop of diluted AgNPs dispersion was held on the copper grid, and one drop of a 0.02 g/mL aqueous solution of phosphotungstic acid was used to contrast the picture enhancement. EDAX (OXFORD XMX N) was employed to study the distribution of elemental composition of MEHNPs. The EDAX detector was used to conduct an elemental analysis in the scanned area [26].
Animals and experimental conditions
Adult albino mice of both sexes (45 male animals for electrochemical convulsions and 3 female for acute toxicity studies), weighing between 20 and 25 g were obtained from Sree Venkateshwara Enterprises with Reg. No. 237/2000/CPCSEA. All the animals were kept under standard laboratory conditions, such as (23±10) ℃ and a relative humidity of about 55%, with a light-dark cycle maintained. For the whole study, all animals had ad libitum access to food and water. The animal experiment was approved (SVCP/IAEC/I-OO6/2018-2019) and conducted in accordance with the Institutional Animal Ethics Committee guidelines and the animal care guidelines of the CPCSEA in New Delhi, India. To prevent bias, the randomized form of animal selection was used for treatments as separate study groups [27]. The doses of Phenytoin, Diazepam, MEME, and administration schedules were chosen based on the previously reported studies [28, 29].
Acute toxicity study of extract
The acute toxicity studies were conceived in accordance with OECD Directives 423. A single oral dose (2000 mg/kg) of ME methanol extract was administered to three female mice [30] and observed individually for gross behavioral, neurologic, and autonomic toxic signs such as changes in heart rate, respiration, salivation, lacrimation, drowsiness, convulsions, motor control, etc [31].
In Vivo Studies
Induction of electro convulsions
For electro convulsions, 20 male animals were divided into four groups of five animals each (n = 5). Group I-Control (NS-0.9% p.o.) + MES (45 mA for 0.2 s); Group II-Phenytoin (25 mg/kg. i.p.) + MES (45 mA for 0.2 s); Group III-MEME-B (200 mg/kg, p.o.) + MES (45 mA for 0.2 s); Group IV-MEHNPs (200 mg/kg, p.o.) + MES (45 mA for 0.2 s). The maximum seizure pattern was induced by an electro convulsiometer (Techno, India), which was supplied via ear-clip electrodes with alternating current (0.2 s stimulation durations and 45 mA). HLE duration was observed [32, 33] before inserting electrodes to allow the induction of electro convulsions, and 0.9% solution of the saline was poured in every ear. Both MEME and MEHNPs were orally administered 45 min before the electroshock was detected and the animals were observed. As a criterion for anti-epileptic behavior relative to control groups [34], the ability of MEME-B and MEHNPs to abolish or minimize the length of the tonic hind limb extensor process was established.
Induction of chemo convulsions
To induce chemo convulsions, 25 male animals were divided into five groups of five animals each
(n = 5). Group I-Control (NS-0.9% p.o.); Group II-PTZ (105 mg/kg i.p.); Group III-Diazepam (2 mg/kg i.p.) + PTZ (105 mg/kg i.p.); Group IVMEME-B (200 mg/kg, p.o.) + PTZ (105 mg/kg i.p.); Group V-MEHNPs (200 mg/kg, p.o.) + PTZ (105 mg/kg i.p.). Mice were injected intraperitoneally with PTZ at a dose of 105 mg/kg, which was its CD97 (97% convulsive dose for the clonic phase) for inducing chemo-convulsions. MEME-B and MEHNPs were given orally 45 min before PTZ therapy, and the animals were monitored for the next 30 min for developing CT seizures. This CT seizure activity was described as clonus of the entire body lasting more than 3 s with a failure of the righting reflex [35, 36].
Morphometric analysis
To determine the effect of ME extracts on experimental animals, the whole body weight of all experimental animals was reported at regular intervals. Furthermore, at the end of the procedure, the entire brain was extracted and measured to determine any changes in brain weight caused by administering Phenytoin, Diazepam, or extract of ME or its nanoformulations.
Assessment of transfer latency (TL)
The animals were placed separately in the plus-maze apparatus, with the head facing the open arm. The time it took each mouse to reach the closed arm for a given set cut-off period of about 90 s was reported as transfer latency. All the animals were exposed to enough trails before the screening day to reduce experimental bias. The first day’s TL was considered acquisition (learning), while subsequent TL recording was considered retention/consolidation memory of experimental animals [37].
Assessment of neurotoxicity
Rotarod and locomotor tests were used to assess ME neurotoxicity. The latency of the animals falling from the spinning bar running at 25 r/min is automatically recorded in the rotarod test. The animals were subjected to a rotating bar before being selected for suitability and ability to hold a rotating bar within a given time limit (90 s off period), while the locomotor score was recorded using the actphotometer (INCO, India) as a standard procedure for 180 s [38].
Estimation of acetylcholinesterase
AChE was estimated according to standard procedures [39]. In brief, 0.4 mL of brain hippocampal
homogenate was added to a test tube containing 2.6 mL of phosphate buffer (0.1 mol/L, pH = 8). To this 100 µL of DTNB reagent was added followed by the addition of 20 µL of acetylthiocholine iodide solution. The change in absorbance was then noted at 412 nm for 5 min by using the calorimeter and results are expressed as µmol/mg protein using the following equation:
![]() (1)
(1)
where, R is the rate in moles of substrate hydrolyzed per min per gram of tissue, ∆A is Change in
absorbance per min, and C0 is Original concentration of tissue (mg/mL).
Estimation of gamma-aminobutyric acid (GABA)
The content of gamma-aminobutyric acid (GABA) was calculated using the Lowe et al.’s method [40]. Isolated brain tissue was immersed in 5 mL of ice-cold trichloroacetic acid (0.1 g/mL). Homogenization was accompanied by centrifugation at 10000 r/min for 10 m at 0 °C to obtain a sample of brain tissue extract. 0.2 mL of 0.14 mol/L ninhydrin solution was applied to 0.1 mL of brain tissue extract along with 0.5 mol/L bicarbonate buffer (pH = 9.95) and held in a water bath at 60 ℃ for 30 min. After cooling enough, the mixture was treated with 5 mL of copper tartrate reagent, which contain 0.16% disodium carbonate, 0.03% copper sulfate, and 0.0329% tartaric acid. After 10 min intervals, fluorescence was recorded at 377/455 nm by using a spectrofluorimeter.
In vivo antioxidant activity
The antioxidant status was performed in brain tissue homogenates. Lipid peroxidation was characterized by measuring the MDA levels [41] whereas superoxide dismutase (SOD) [42] and catalase (CAT) [43] were measured according to the standard protocols.
Histopathological studies
After the experimental period, animals were sacrificed using a cervical dislocation procedure and
vital organs; for example, the entire brain was isolated for histopathological examinations. Half of each animal's brain was fixed in 10% formalin for twenty four hours followed by washing and subjected to paraffin embedding. The coronal slices of 10 µm across the hippocampus were sliced, mounted, and stained with hematoxylin and eosin (H&E) before being examined under microscopes at various magnifications [44]. Degenerative changes in neuron-like cytoplasmic vacuolation, nuclear chromatin clumping, and fragmentation, among other things, were observed and recorded during the assessment.
Statistical analysis
Statistical analysis was conducted by one-way analysis of variance (ANOVA) followed by Dunnett’s test for multiple comparisons using Graph Pad Prism 5.00® software (GraphPad Software, Inc., San Diego, CA, USA). Values are shown as mean ± SEM. P < 0.05 was treated as significant.
Results
Phytochemical studies
Preliminary phytochemical studies of ME bark extract reveal the presence of various phytochemicals such as terpenoids, flavonoids, tannins, steroids, and saponins.
FT-IR study
FTIR study was performed for both MEME-B (Fig. 1(a)) and MEHNPs (Fig. 1(b)). The results revealed that minor shifts in peaks could be due to the capping, reduction, and stabilization of MEHNPs. The presence of functional groups capping the MEHNPs was confirmed by the FTIR study. Peaks at 3394.2 cm–1 in the bark extract spectrum indicate bonded hydroxyl groups. The —CH stretching vibration of CH2 groups is visible in the peaks at 2920 cm–1 and 2852 cm-1. Furthermore, the C=C stretching vibration is reflected by the peaks at 1603 cm–1 and 2922cm–1. The aromatic ring system’s C–H deformation vibration is reflected by the peaks at 1247 cm–1 and 1027 cm–1.
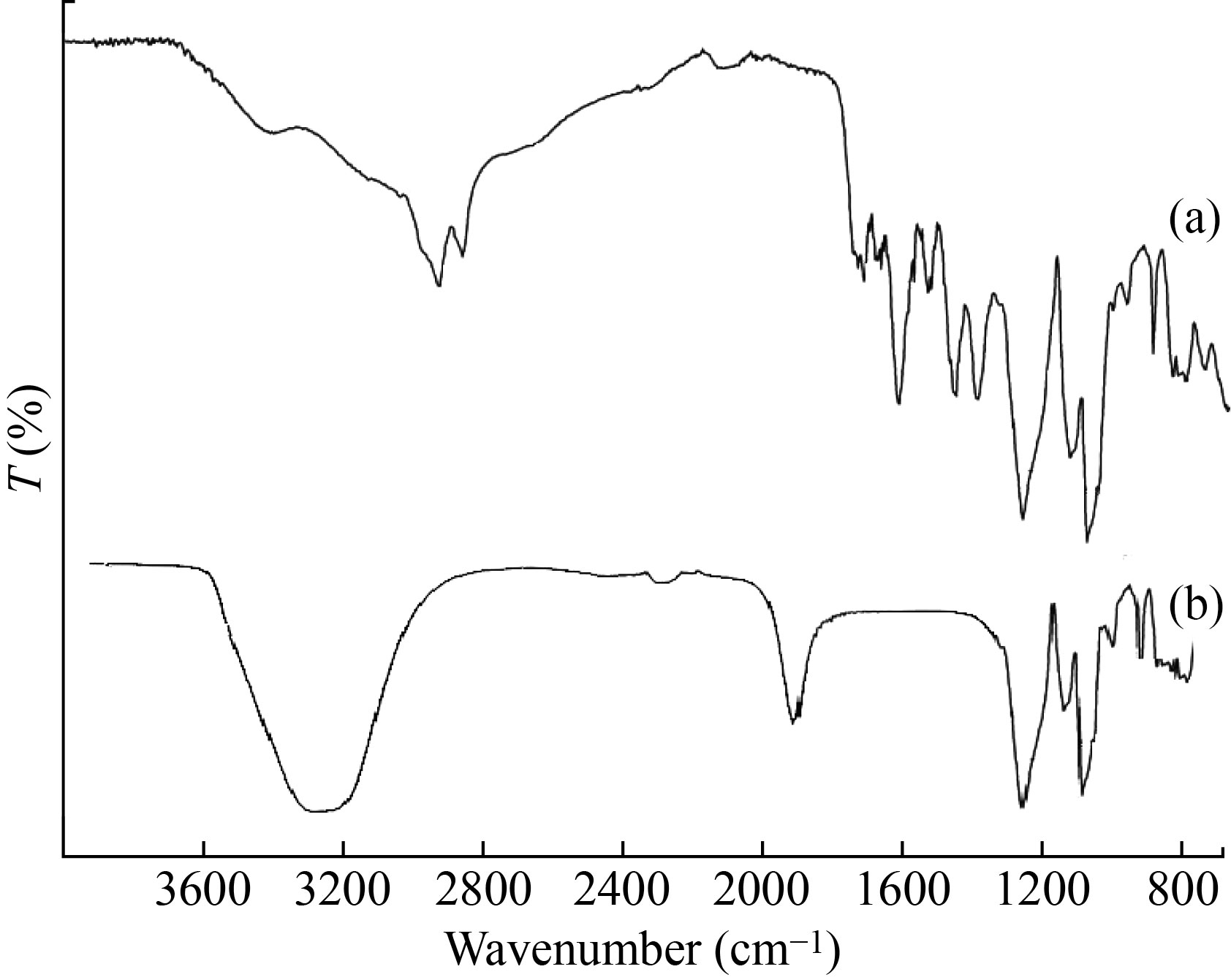
Fig. 1 FT-IR spectra of (a) MEME-B and (b) MEHNPs
HPLC analysis
The prepared MEME-B was qualitatively analyzed by comparing the normal HPLC spectra of ursolic acid (Fig. 2). The retention times for standard ursolic acid (Fig. 2(a)) and ME bark extracts (Fig. 2(b)) were 7.91 and 7.82 min, respectively, in HPLC chromatograms. By comparing the retention time of reference ursolic acid, the identity of the extract's peak was discovered. The presence of ursolic acid was verified by the bark extract.
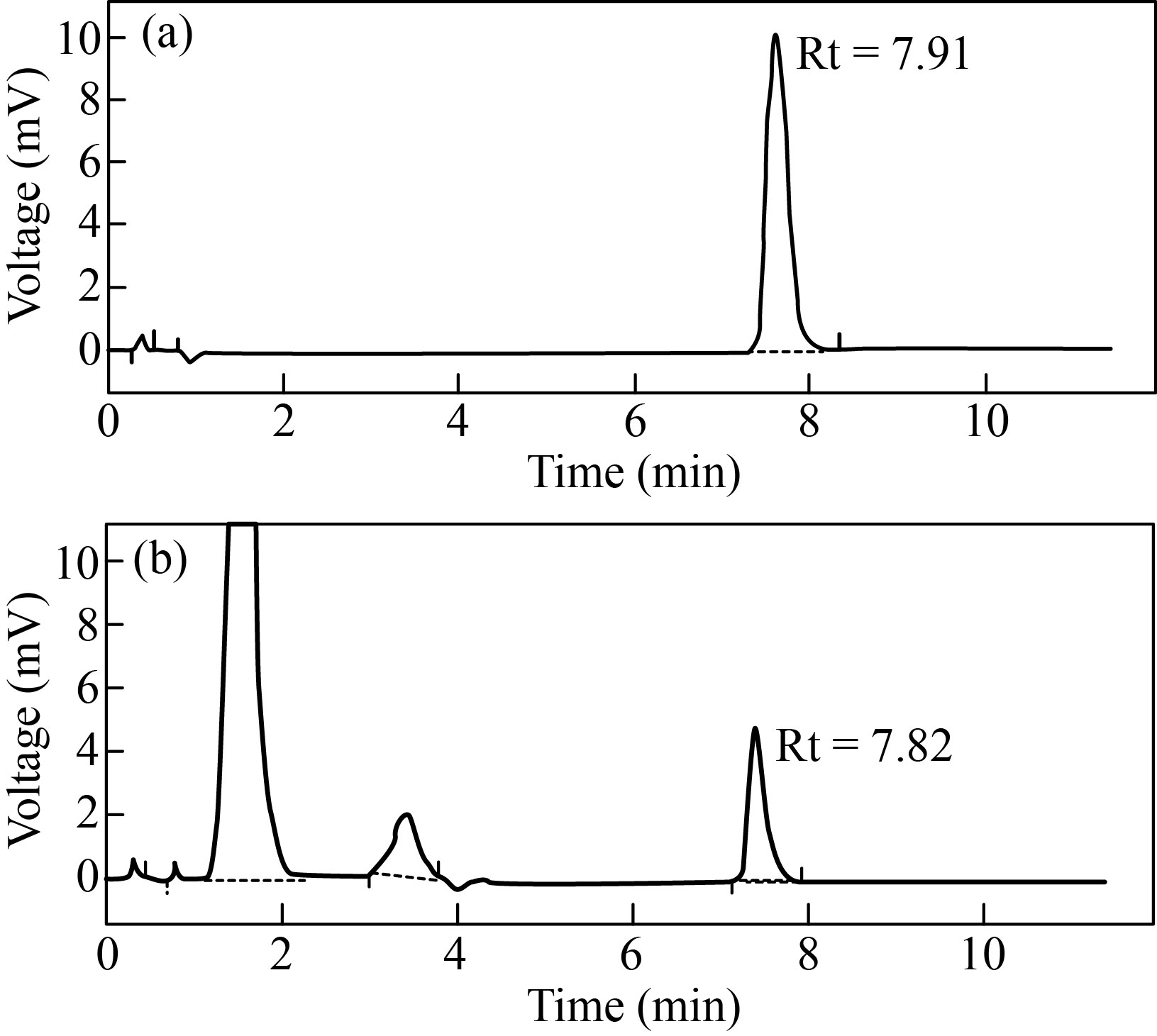
Fig. 2 HPLC chromatogram: (a) standard ursolic acid (Rt = 7.91); (b) MEME-B containing ursolic acid (Rt = 7.82)
Acute toxicity study
Acute toxicity tests revealed that both extract and nonoformulation-treated animals tolerated a maximum dose of 2000 mg/kg body weight with no toxic effects such as changes in heart rate, respiration, salivation, lacrimation, drowsiness, convulsions, and motor coordination. Furthermore, the behavior of ME treated animals appeared normal. Hence, 1/10 of the maximum-tolerated dose (200 mg/kg body weight) was chosen for the study.
Characterization of nanoparticles
According to the results, MEHNPs were found to have a particle size of 24 nm. Figure 3(a) depicts the size distribution histogram of complex light scattering of nanoparticles. The surface charge, as well as the stability of the prepared compounds, is normally reflected in the zeta potential. Nanoparticles with zeta potentials greater than 30 mV is normally more stable in suspension and have a higher surface charge, which prevents particle aggregation. The zeta potential of MEHNPs was found to be –29.5 mV (Fig. 3(b)) in this analysis.
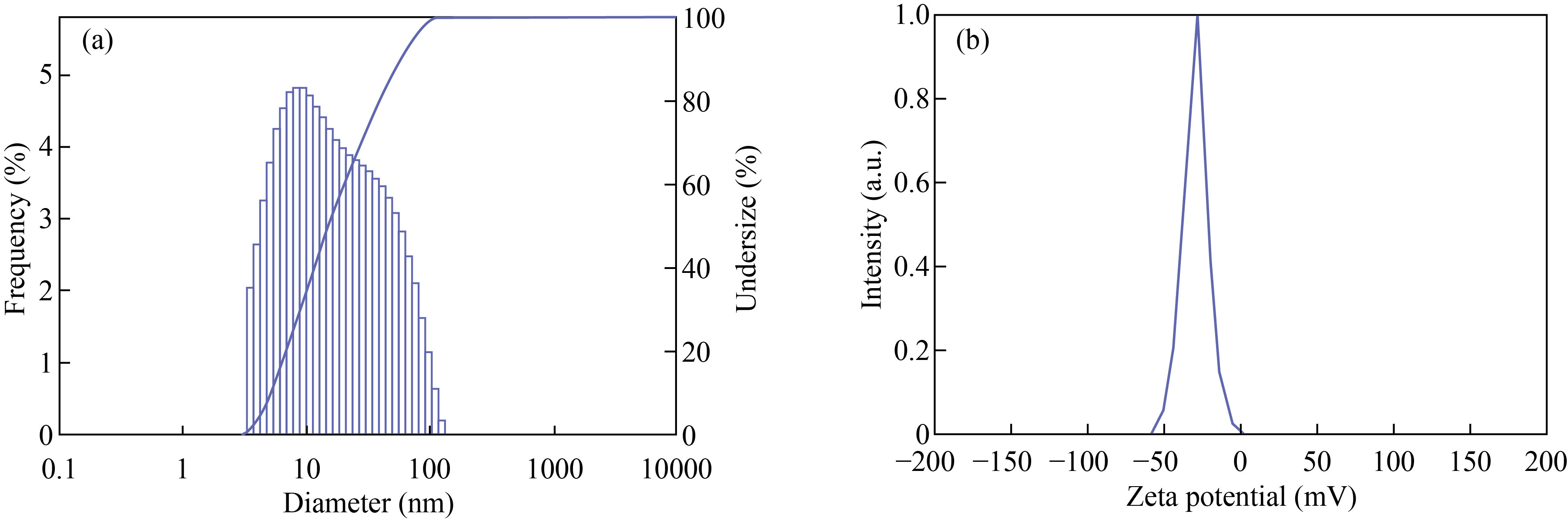
Fig. 3 Particle size analysis of (a) MEHNPs and (b) zeta potential of MEHNPs
The size and shape of the silver nanoparticles synthesized in the study (Fig. 4(a)) were confirmed using TEM (Fig. 4(b)). The results show that the synthesized nanoparticles are nanosize and spherical, and that they are ideal in the size range. Figure 4(c) depicts the energy dispersion X-ray spectra (EDAX) of MEHNPs. Ag, C, and O were discovered in the EDAX of MEHNPs. However, no N signal was detected from MEHNPs, and C and O signals are emitted by organic compounds in ME bark extract, implying that some of the extract is encasing the herbal nanoparticles.
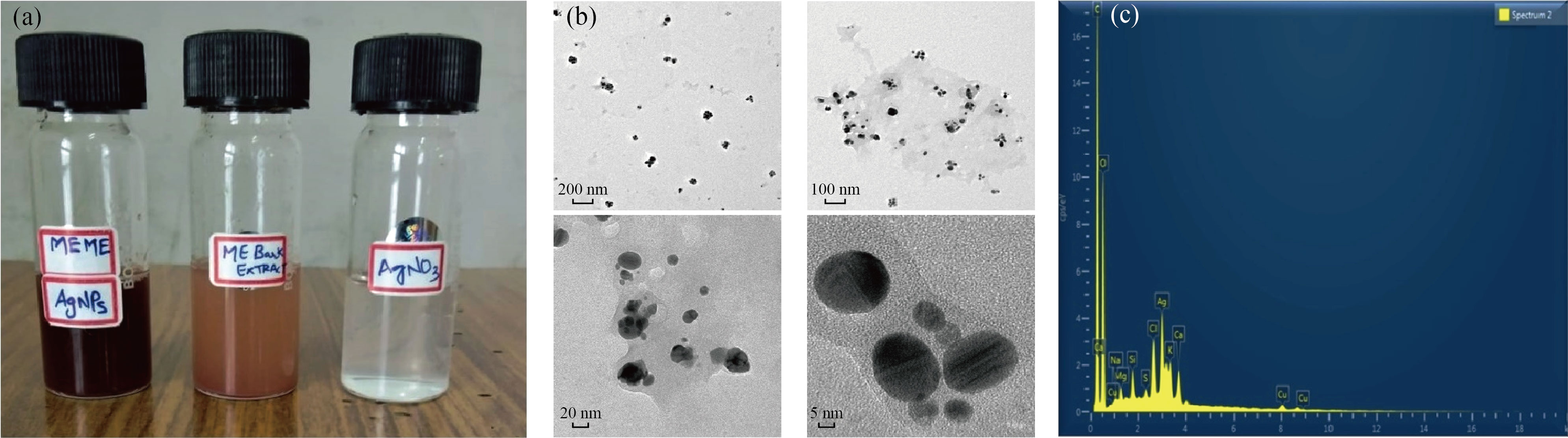
Fig. 4 (a) Synthesized nanoparticles of Mimusops elengi; (b) TEM analysis of MEHNPs using different magnifications; (c) EDAX analysis of MEHNPs
In Vivo Study
Morphometric assessment
To assess the toxicity of ME, the body weights of all experimental animals were measured at regular intervals throughout the study. According to the findings, there was a substantial difference in weight between the control and experimental classes. MEME-B and MEHNP-treated groups also displayed a substantial increase in body weight (* P < 0.05). Similarly, at the end of the experimental study, brain weight was measured; results showed that there was no difference in brain weight between extract and nanoformulation-treated groups in both electro and chemo convulsion models in mice (Table 1).
Table 1 Effect of MEME-B and MEHNPs on morphometric analysis in electro convulsions
Treatments | Bodyweight (g) | Brain weight (g) |
Control (NS-0.9% p.o.) + MES (45 mA for 0.2 s) | 26.8 ± 1.03 | 0.36 ± 0.05 |
Phenytoin (25 mg/kg. i.p.) + MES (45 mA for 0.2 s) | 32.3 ± 0.85** | 0.35 ± 0.03 |
MEME-B (200 mg/kg, p.o.) + MES (45 mA for 0.2 s) | 31.2 ± 1.04* | 0.36 ± 0.04 |
MEHNPs (200 mg/kg, p.o.) + MES (45 mA for 0.2 s) | 31.5 ± 0.91* | 0.35 ± 0.04 |
Note: Number of animals (n = 5); Values are mean ± SEM: * P < 0.05, **P < 0.01 and ***P < 0.001 when compared with control groups; ns: non significant.
MES induced convulsions
The results of the MES model show a significant differential reduction of hind limb extension (* P < 0.05) phase in both the MEME-B and MEHNPs groups, as shown in Fig. 5(a). Similarly, the same pattern of convulsion threshold reduction (***P < 0.001) was observed in the standard drug PHT (25 mg/kg. i.p.) treated animals. The study's findings also show that MEHNP-treated groups had the highest percentage of mortality reduction (20%) compared with control groups (80%), while MEME-B-treated groups had 40% mortality (data not shown) in the study.
PTZ induced convulsions
The onset of clonus convulsion and its duration was recorded in all experimental animals in the PTZ model. As shown, pretreatment with MEME-B had a protective effect (* P < 0.05) on duration of CT seizures (Fig. 5(b)) in mice, as well as a delay in the occurrence of CT convulsions (Fig. 5(c)).
Similarly, MEHNP-treated animals had a significantly shorter length of CC (**P < 0.01) and a significantly longer onset of CC (**P < 0.01) than control animals, as shown in Figs. 5(b) and 5(c). From the results, pretreatment with both MEME-B and MEHNPs (200 mg/kg p.o.) showed a protective effect ( * P < 0.05) upon the duration of the CT type of seizure along with the delay of onset of CT convulsions. MEHNP treated group showed better responses to the reduction in the duration of CC (**P < 0.01) and significantly longer onset of CC (**P < 0.01) when compared with control and MEME-B treated groups (* P < 0.05).
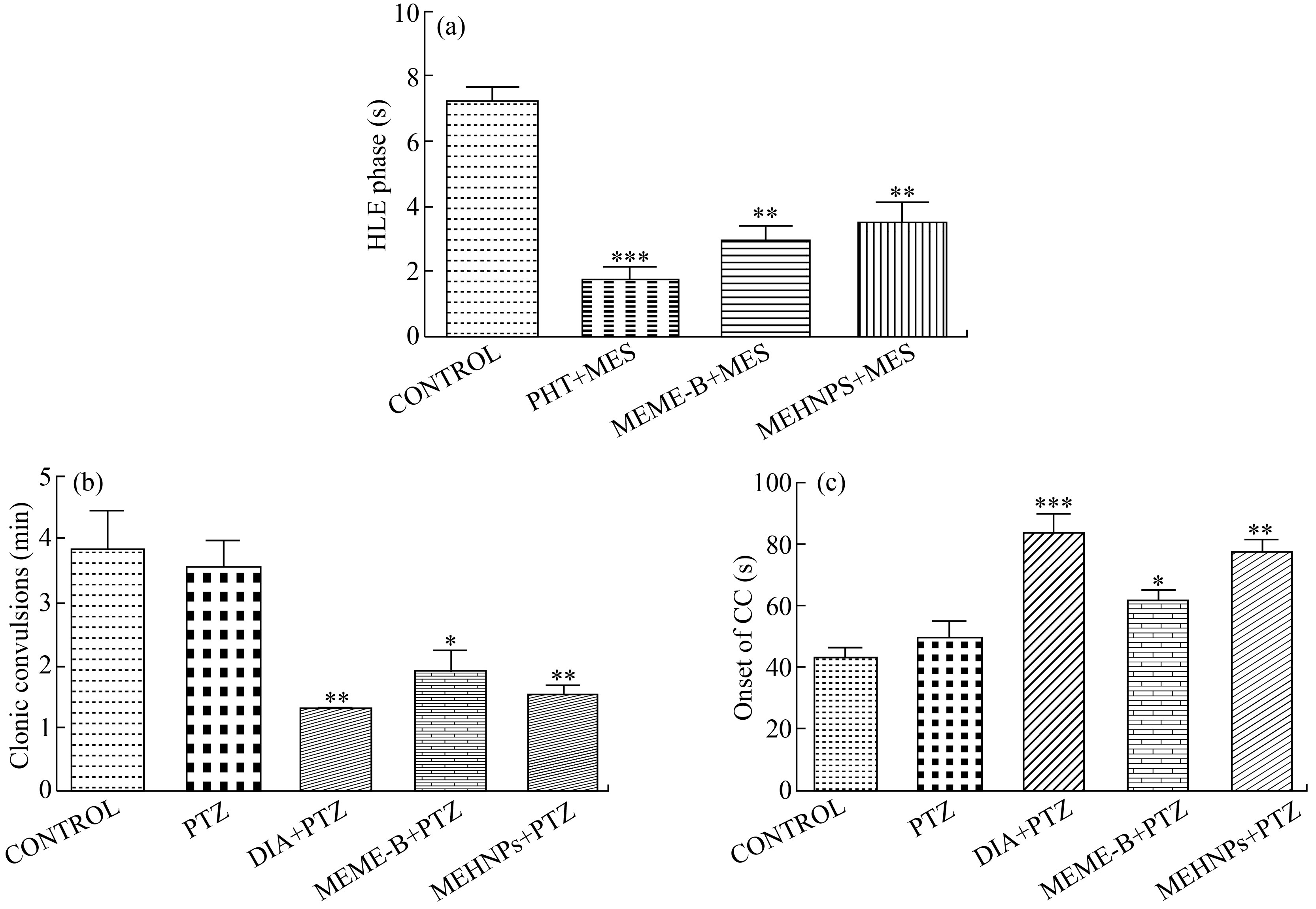
Fig. 5 Effect of MEME-B and MEHNPs on MES and PTZ induced convulsions (Number of animals (n = 5): Values are mean ± SEM; * P < 0.05, **P < 0.01 and ***P < 0.001 when compared with control and PTZ treated groups; ns: non significant)
Assessment of neurotoxicity
In the rota rod experiment, there were significant changes in the fall of time; whereas in the case of an actophotometer study, changes in locomotion scores were considered as criteria to check the neurotoxicity. From the results, no significant changes were observed in both fall of time (Fig. 6(a)) and locomotion score (Fig. 6(b)) among the experimental groups throughout the study, which indicates and confirms the lack of neurotoxic property of both MEME-B and MEHNPs at the dose of 200 mg/kg, p.o.
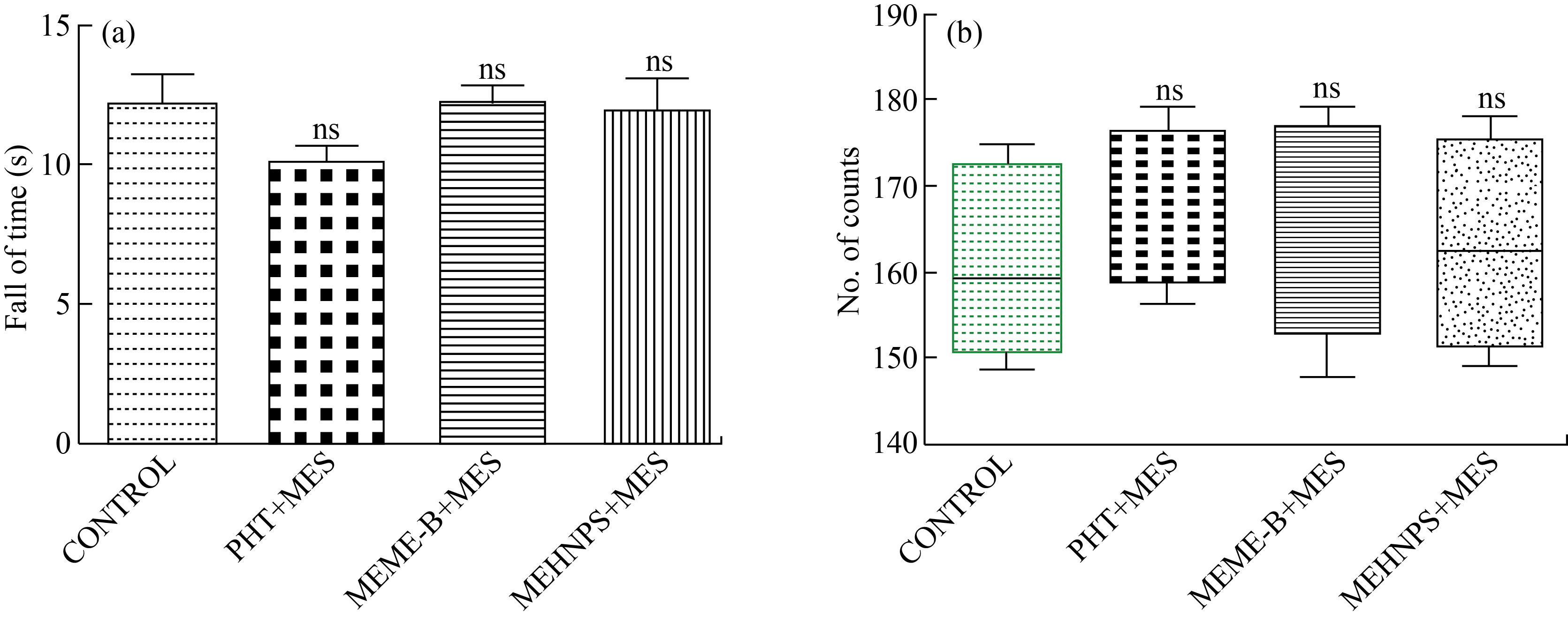
Fig. 6 Assessment of neurotoxicity in MES induced electro convulsion in mice: (a) Fall of time in rotarod test; (b) Locomotion score (No. of counts) in actophotometer test (Number of animals (n = 5): Values are mean ± SEM: * P < 0.05, **P < 0.01 and ***P < 0.001 when compared with control groups; ns: non significant)
Transfer latency
As depicted in Table 2, both in PTZ and MES models, animals showed a significant increase in transfer latency, whereas both MEME-B (200 mg/kg p.o.) and MEHNPs significantly reduced (*P < 0.05) these impairments on mice with a significant increase in open arm entry and time spent in the open arm, indicating the anti-anxiety potential of ME.
Table 2 Effect of MEME-B and MEHNPs on transfer latency in elevated plus maze test in mice
Treatments | Transfer latency (s) | Time spent (s) |
Control (NS-0.9% p.o.) + MES (45 mA for 0.2 s) | 5.36 ± 0.16 | 20.5 ± 2.04 |
Phenytoin (25 mg/kg. i.p.) + MES (45 mA for 0.2 s) | 16.44 ± 0.23*** | 42.0 ± 1.57*** |
MEME-B (200 mg/kg, p.o.) + MES (45 mA for 0.2 s) | 8.04 ± 0.13*** | 33.6 ± 1.07*** |
MEHNPs (200 mg/kg, p.o.) + MES (45 mA for 0.2 s) | 7.90 ± 0.36*** | 29.5 ± 1.35*** |
Note: Number of animals (n = 5); Values are mean ± SEM; * P < 0.05, **P < 0.01 and ***P < 0.001 when compared with control groups; ns: non significant.
Neurochemical estimations
In this study, neurochemical estimates were made to determine whether there were any changes in the levels of most important inhibitory neurotransmitters like GABA and enzymes like acetylcholinesterase (AChE), which is a key enzyme responsible for the breakdown of one of the important excitatory neurotransmitter, i.e, acetylcholine (ACh). Obtained results confirm that a significant rise in AChE, GABA activity in MEME-B ( * P < 0.05) and MEHNP groups (**P < 0.01) groups are shown in Table 3. Therefore, the antiepileptic activity of both MEME-B and MEHNP could be attributed to inhibition of excitatory neurotransmission by increasing the level of AChE and enhancement of inhibitory neurotransmission by increasing the level of GABA.
Table 3 Effect of MEME-B and MEHNPs on neurochemicals in chemo convulsions in mice
Treatments | Acetylcholinestrase (AChE) (nmol/mg protein) | GABA (ng/g of brain tissue) |
Control (NS-0.9% p.o.) + PTZ (105 mg/kg i.p.) | 1.09 ± 0.11 | 39.8 ± 4.01 |
PTZ (105 mg/kg i.p.) | 0.67 ± 0.19 | 24.0 ± 2.80 |
Diazepam (2 mg/kg i.p.) + PTZ | 1.79 ± 0.19* | 60.5 ± 4.98** |
MEME-B (200 mg/kg, p.o.) + PTZ | 1.68 ± 0.07* | 54.4 ± 3.82* |
MEHNPs (200 mg/kg, p.o.) + PTZ | 1.80 ± 0.08** | 58.8 ± 1.48** |
Note: Number of animals (n = 5); Values are mean ± SEM; * P < 0.05, **P < 0.01 and ***P < 0.001 when compared with control groups; ns: non significant.
Antioxidant Activity
According to the findings of the antioxidant analysis, PTZ administration lowered brain antioxidant status by lowering SOD and CAT levels but increasing LPO levels. The administration of MEME-B and MEHNPs, on the other hand, substantially increased antioxidant enzyme levels (* P < 0.05) and scavenged the free radicals that caused the impairments (Table 4). Compared with MEME-B treated groups, MEHNP treated groups showed significantly better results in terms of antioxidant enzyme levels and LPO reduction.
Table 4 Effect of MEME-B and MEHNPs on invivo antioxidant activity in chemo convulsions model in mice
Treatments | LPO (nmol of MDA/mg of protein) | SOD (µ/mg of protein) | CAT (U/mg of protein) |
Control (NS-0.9% p.o.) + PTZ (105 mg/kg i.p.) | 0.06 ± 0.01 | 39.87 ± 6.80 | 2.31 ± 0.15 |
PTZ (105 mg/kg i.p.) | 0.40 ± 0.09 | 33.6 ± 4.18 | 0.75 ± 0.15 |
Diazepam (2 mg/kg i.p.) + PTZ | 0.13 ± 0.04 | 41.7 ± 7.37* | 3.80 ± 0.22** |
MEME-B (200 mg/kg, p.o.) + PTZ | 0.36 ± 0.01* | 50.9 ± 8.25** | 3.47 ± 0.31* |
MEHNPs (200 mg/kg, p.o.) + PTZ | 0.21 ± 0.04** | 62.7 ± 8.05** | 3.93 ± 0.31** |
Note: Number of animals (n = 5); Values are mean ± SEM; * P < 0.05, **P < 0.01 and ***P < 0.001 when compared with PTZ groups; ns: non significant.
Histopathological studies
Histopathological examination of brain tissue (Hand E, 100 X: Stained mice brain – Hippocampus slice) demonstrating the protective effect of ME extracts and its nanoparticles (MEHNPs) on PTZ-induced degeneration and tissue necrosis (Fig. 7). The cellular level changes in the brain tissue were shown by the black arrow compared with control (Fig. 7(a)). Compared with PTZ-treated animals (Fig. 7(b)), MEME (Fig. 7(d)) and MEHNPs (Fig. 7(e)) showed the least gliosis and lack of neurodegeneration with mild injury, implying and supporting its protective function. Injecting PTZ caused seizures that increased the density of dark neurons in the hippocampus area of the brain, which is the characteristic of black arrow. Pretreatment with MEME and MEHNPs, on the other hand, shielded hippocampus cells from neuron degeneration and tissue necrosis. A few hippocampus cells were found to have degenerated, while the diazepam-treated (Fig. 7(c)) community showed normal hippocampus cells.
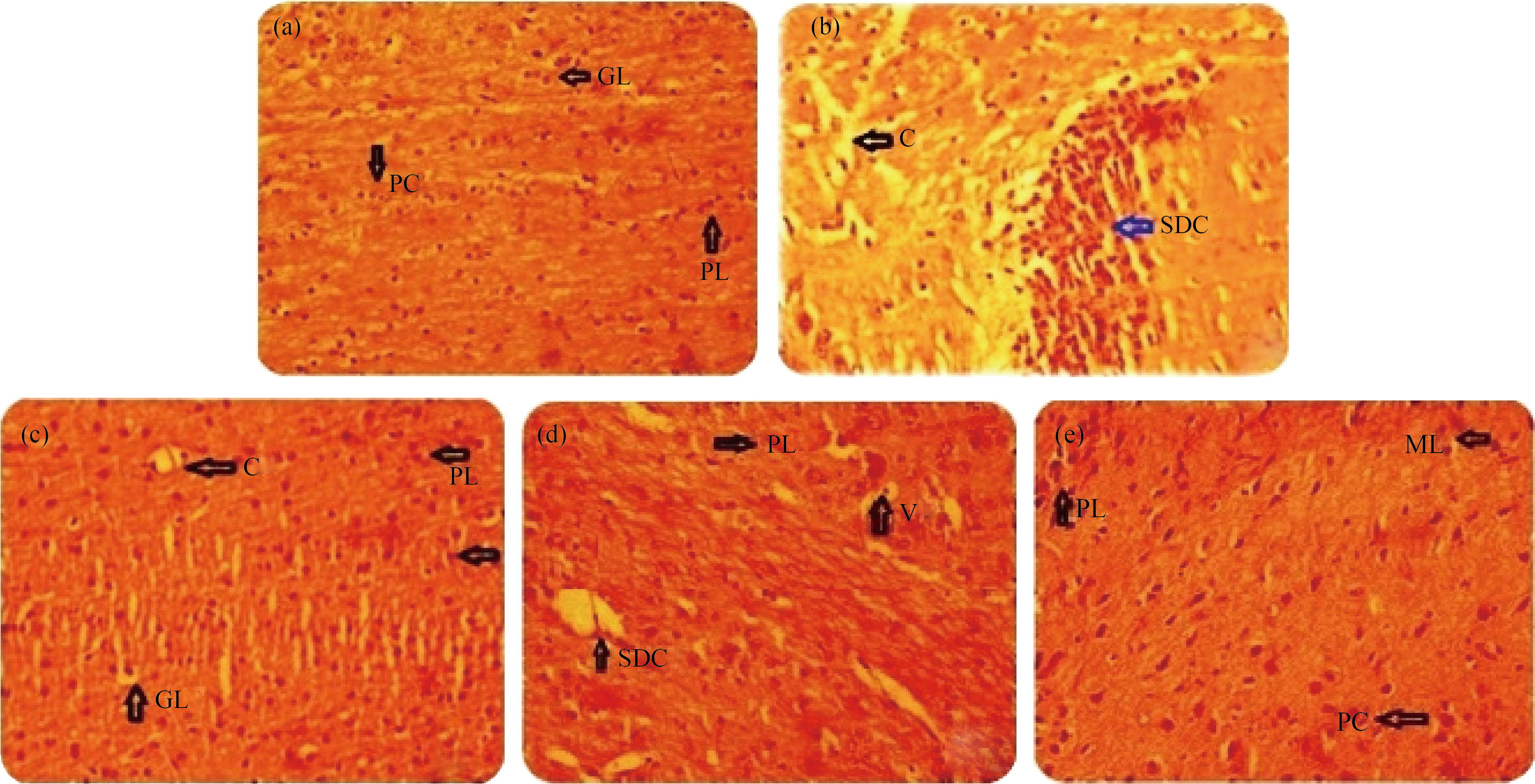
Fig. 7 Histopathological examination of the brain tissues of PTZ induced chemo convulsion in mice: (a) Control; (b) PTZ; (c) Diazepam; (d) MEME-B; (e) MEHNPs; (C: Congestion; SDC: Structural Degenerative Changes; GL: Granular Layer; ML: Molecular Layer; PC: Purkinje Cells; PL: Purkinje Layer; V: Vacuolization; WM: White Matter)
Discussion
The most important characteristics of nanoparticle systems are particle size and size distribution. The nanoparticle systems in in-vivo distribution, biological fate, and toxicity can be determined by the size of the particle. They may also affect drug loading, drug release, and nanoparticle stability [45]. TEM results of the present study indicate the particle size of ME bark was about 24 nm which was found to be the optimal range for better delivery of particles. In the present investigation, MEME-B and MEHNPs were prepared and characterized using standard methods. The presence of alkane, alkene, aldehyde, ether ester, and aromatic phytoconstituents was verified using FTIR spectroscopy. Ursolic acid is discovered in the bark extract after further HPLC analysis. Hence, results of this study may provide important information for further ethno pharmacological investigations.
Results from acute toxicity test revealed that both extract and nonoformulation-treated animals were healthy, with no changes in behavior with no toxic effects noticed. In addition, morphometric parameters such as body weights were calculated regularly and brain weights were measured at the end of the study to assess ME negative effects. The results showed a substantial increase in body weight (*P < 0.05), indicating that MEME-B and MEHNPs are both safe and beneficial. There were no differences in brain weight analysis between the experimental groups. Furthermore, results from the actophotometer and rotarod tests show that no substantial differences in locomotion and time fall were observed among all experimental groups, indicating that ME, both in extract type and nanoformulations, has no neurotoxic effects among the animals.
The results of the MES model show a significant reduction in HLE phase in MEME-B and MEHNP groups, as well as the highest percentage of mortality security (80%) in MEHNP groups compared with the control groups (20%), while MEME-B treated groups.
In this study, to assess cognitive parameters, all experimental animals were subjected to multiple cognitive assessment trials. In an elevated plus-maze test, a large increase in TL was observed after electro and chemo-convulsion, while MEME-B and MEHNPs substantially reduced these impairments in mice, demonstrating the animal possible learning capability. There was also a significant rise in open arm entries and time spent in the open arm, indicating that ME has anti-anxiety properties.
An imbalanced antioxidant defense mechanism, as well as the overproduction or introduction of free radicals from the atmosphere into the living system, results in serious consequences, including Alzheimer’s disease, Parkinson’s disease, aging, and many other neural disorders. Aside from other environmental or genetic causes, oxidative stress (OS) resulting in free radical assault on neural cells plays a disastrous role in neuro-degeneration. According to studies, an imbalance between the antioxidant system and oxidative stress can cause various diseases, including epilepsy [46, 47]. Hence, the antioxidant activity was measured in the current study to determine the protective nature and effect on antioxidant systems. According to the findings, PTZ administration decreased brain antioxidants such as SOD and CAT, and raised the amount of lipid peroxidation (LPO), while MEME-B and MEHNP administration substantially increased antioxidant enzyme levels and scavenged the free radicals responsible for the impairments. However, when compared with MEME classes, animals given MEHNPs showed greater increases in antioxidant enzyme levels and a decrease in LPO.
Furthermore, according to available data, imbalances in excitatory and inhibitory neurotransmitters in the CNS can trigger and provoke various forms of convulsions [48]. Any decrease in GABA levels commonly causes seizures because it is one of the most powerful inhibitory neurotransmitters in the CNS. Several anticonvulsant drugs on the market work by increasing GABA neurotransmission through various mechanisms, such as blocking the enzyme GABA transaminase or inhibiting GABA reuptake into presynaptic nerve endings [49]. In this study, results from neurochemical estimations confirm that a significant rise in AChE, GABA activity in MEME-B (*P < 0.05) and MEHNP groups (**P < 0.01) groups. Therefore, the antiepileptic activity of both MEME-B and MEHNP could be attributed to inhibition of excitatory neurotransmission by increasing the level of AChE and enhancement of inhibitory neurotransmission by increasing the level of GABA. The results were further confirmed by histopathological tests, which revealed that MEHNPs had less gliosis and a lack of neurodegeneration with mild damage compared with PTZ-treated animals, implying that MEHNPs may play a protective role.
Conclusion
In the present study, MEME-B and MEHNPs were prepared and characterized using standard methods. Both extract and nonoformulation-treated animals were healthy, with no changes in behavior. The results of neurochemical analyses show a significant rise in Acetylcholinesterase (AChE) and Gamma Butyric acid (GABA) levels. MEME and MEHNPs increased antioxidant enzyme levels and scavenged free radicals to significant extent. Histological findings revealed the least gliosis and a major reversal of the normal neuronal cell architecture. In conclusion, both MEME-B and MEHNP have demonstrated significant antiepileptic properties in both models, which can be attributed to suppressing excitatory neurohumoral transmission and increasing inhibitory neurotransmission while significantly improving antioxidant status. In contrast to MEME-B, MEHNPs have demonstrated improved oxidative stress reduction and modification of neurochemicals such as AChE and GABA in the form of nanoformulation.
List of Abbreviations
MEHNPs: Mimusops elengi herbal nanoparticles; MEME-B: methanol extract of Mimusops elengi bark; CPCSEA: Committee for the Purpose of Control and Supervision of Experiments on animals; ANOVA: oneway analysis of variance; PTZ: Pentylenetetrazole; CD97: Convulsive Dose; EC: electro convulsions; CC: Chemo Convulsions; HLE: hind limb extension; TL: Transfer latency; AChE: Acetylcholinesterase; GABA: Gamma Butyric acid; LPO: Lipid Peroxidation.
Acknowledgments
The authors gratefully thank the management of Sree Vidyanikethan Educational Trust (SVET) for providing the required facilities and support.
Conflict of interests
The authors declare that no competing interest exists.
References
[1] O. Devinsky, A. Vezzani, T.J. O’Brien, et al. Epilepsy. Nature Reviews Disease Primers, 2018, 4: 18024. https://www.nature.com/articles/nrdp201824
[2] R. Jayaraman, K.T. Manisenthil, T. Anitha, et al. Influence of etoricoxib on anticonvulsant activity of phenytoin and diazepam in experimental seizure models in mice. Journal of Pharmacy and Pharmacology, 2010, 62(5): 610–614. https://doi.org/10.1211/jpp.62.05.0008
[3] K.M. Fiest, K.M. Sauro, S. Wiebe, et al. Prevalence and incidence of epilepsy: A systematic review and metaanalysis of international studies. Neurology, 2017, 88(3): 296–303. https://doi.org/10.1212/wnl.0000000000003509
[4] W.A. Hauser, E. Beghi. First seizure definitions and worldwide incidence and mortality. Epilepsia, 2008, 49: 8-12. https://doi.org/10.1111/j.1528-1167.2008.01443.x
[5] D. McCorry, D. Chadwick, A. Marson. Current drug treatment of epilepsy in adults. The Lancet Neurology, 2004, 3(12): 729–735. https://doi.org/10.1016/s1474-4422(04)00935-4
[6] W. Löscher, D. Schmidt. Modern antiepileptic drug development has failed to deliver: Ways out of the current dilemma. Epilepsia, 2011, 52(4): 657–678. http://dx.doi.org/10.1111/j.1528-1167.2011.03024.x
[7] B. Bauer, J. Schlichtiger, A. Pekcec, et al. The blood brain barrier in epilepsy. Clinical and genetic aspects of epilepsy. London: IntechOpen, 2011: 23-54. https://doi.org/10.5772/21561
[8] A.K. Ngugi, C. Bottomley, G. Fegan, et al. Premature mortality in active convulsive epilepsy in rural Kenya: Causes and associated factors. Neurology, 2014, 82: 582–589. https://doi.org/10.1212/wnl.0000000000000123
[9] R. Sridharan. Epidemiology of epilepsy. Current Science, 2002, 82(6): 664-670. https://www.jstor.org/stable/24106692
[10] M.S. Baliga, R.J. Pai, H.P. Bhat, et al. Chemistry and medicinal properties of the Bakul (Mimusops elengi Linn): A review. Food Research International, 2011, 44(7): 1823–1829. https://doi.org/10.1016/j.foodres.2011.01.063
[11] P. Kadam, K. Yadav, R. Deoda, et al. Mimusops elengi: A review on ethnobotany, phytochemical and pharmacological profile. Journal of Pharmacognosy and Phytochemistry, 2012, 1(3): 64–74. https://www.phytojournal.com/vol1Issue3/Issue_sept_2012/10.1.pdf
[12] M. Jerline, G. Jothi. Effect of Mimusops elengi Linn. bark extract on alloxan induced hyperglycemia in albino rats. Journal of Cell and Tissue Research, 2009, 3(1): 56–62.
[13] R. Raghunathan, R. Mitra. Pharmacognosy of Indigenous drugs. New Delhi, India: Central Council for Research in Ayurveda and Siddha, 1982: 484–510.
[14] H. Du, X.Q. Chen. A comparative study of the separation of oleanolic acid and ursolic acid in Prunella vulgaris by high-performance liquid chromatography and cyclodextrin-modified micellar electrokinetic chromatography. Journal of the Iranian Chemical Society, 2009, 6(2): 334–340. http://dx.doi.org/10.1007/BF03245842
[15] A. Shirwaikar, S. Prabu, G, Kumar. Herbal excipients in novel drug delivery system. International Journal of Comprehensive Pharmacy, 2008, 70(4): 415–422. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2792536/
[16] A.T. Sharma. S. Mitkare, R.S. Moon, et al. Multi component herbal therapy: A review. International Journal of Pharmaceutical Sciences Review and Research, 2011, 6: 185–187.
[17] M.A. Rossi. Targeting anti-epileptic drug therapy without collateral damage: Nanocarrier-based drug delivery. Epilepsy Currents, 2012, 12(5): 199–200. https://doi.org/10.5698/1535-7511-12.5.199
[18] G.D. Anderson, R.P. Saneto. Current oral and non oral routes of antiepileptic drug delivery. Advanced Drug Delivery Reviews, 2012, 64: 911-918. https://doi.org/10.1016/j.addr.2012.01.017
[19] G. Modi, V. Pillay, Y. E . Choonara , et al . Nanotechnological applications for the treatment of neurodegenerative disorders. Progress in Neurobiology, 2009, 88: 272–285. https://doi.org/10.1016/ j.pneurobio.2009.05.002
[20] C.K. Kokate, A.P. Purohit, S.B. Gokhale. A textbook of pharmacognosy. 4th edition. New Delhi: CB Publishers, 2006: 93–597.
[21] H.O. Edeoga, D.E. Okwu, B.O. Mbaebie. Phytochemical constituents of some Nigerian medicinal plants. African Journal of Biotechnology, 2005, 4: 685–688. https://doi.org/10.5897/ajb2005.000-3127
[22] P.V. Kadam, R. Deoda, R.S. Shivatare, et al. Pharmacognostic, phytochemical and physiochemical studies of Mimusops Elengi Linn stem bark (Sapotaceae). Der Pharmacia Lettre, 2012, 4(2): 607–613.
[23] V. Dighe, D. Mestry. High performance liquid chromatographic method for simultaneous quantitation of betulinic acid and ursolic acid from dried stem bark powder of Mimusops elengi Linn. International Journal of Research in Ayurveda and Pharmacy, 2013, 4(6): 899–902. https://doi.org/10.7897/2277-4343.04625
[24] S. Ahmed, Saifullah, M. Ahmad, et al. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. Journal of Radiation Research and Applied Science, 2016, 9(1): 1–7. https://doi.org/10.1016/j.jrras.2015.06.006
[25] N.N. Palei, B.C. Mohanta, M.K. Das, et al. Lornoxicam loaded nanostructured lipid carriers for topical delivery: Optimization, skin uptake and in vivo studies. Journal of Drug Delivery Science and Technology, 2017, 39: 490–500. http://dx.doi.org/10.1016/j.jddst.2017.05.001
[26] N.N. Palei, S. Ramu, V. Vijaya. Green synthesis of silver nanoparticles using leaf extract of Lantana camara and its antimicrobial activity. International Journal of Green Pharmacy, 2020, 14(2): 152–161. https://doi.org/10.22377/ijgp.v14i02.2878
[27] J.F. Zolman. Biostatistics: Experimental design and statistical inference. New York: Oxford University Press, 1993: 343.
[28] J. Rajangam, O. Lavanya. Effect of Rosuvastatin on learning and memory in scopolamine induced amnesia in Mice. Trends in Medicine, 2018, 18: 1–4. https://doi.org/10.15761/tim.1000135
[29] P. Ashok, B.C. Koti, A.H. Vishwanathswamy. Antiurolithiatic and antioxidant activity of Mimusops elengi on ethylene glycol-induced urolithiasis in rats. Indian Journal of Pharmacology, 2010, 42(6): 380–383.
[30] O. Bedi, P. Krishan. Investigations on acute oral toxicity studies of purpurin by application of OECD guideline 423 in rodents. Naunyn-Schmiedeberg’s Arch Pharmacol, 2020, 393(4): 565–571. https://doi.org/10.4103/0253-7613.71925
[31] Organization for Economic Coopera tion and Development. Guideline for testing of chemicals: Acute oral toxicity - fixed dose procedure. OECD, 2001: 1–14.
[32] S. Ramu, A. Murali, A. Jayaraman. Phytochemical screening and toxicological evaluation of Sargassum wightii greville in wistar rats. Turkish Journal of Pharmaceutical Sciences, 2019, 16(4): 466–475. https://doi.org/10.4274/tjps.galenos.2018.68442
[33] N. Rathor, T. Arora, S. Manocha, et al. Anticonvulsant activity of Aloe vera leaf extract in acute and chronic models of epilepsy in mice. Journal of Pharmacy and Pharmacology, 2014, 66(3): 477–485. https://doi. org/10.1111/jphp.12181
[34] W. Löscher, D. Schmidt. Which animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Journal of Epilepsy Research, 1988, 2: 145–181. http://dx.doi.org/10.1016/0920-1211(88)90054-X
[35] S.N. Mandhane, K. Aavula, T. Rajamannar. Timed pentylenetetrazol infusion test: A comparative analysis with s.c.PTZ and MES models of anticonvulsant screening in mice. Seizure, 2007, 16(7): 636–644. http://dx.doi.org/10.1016/j.seizure.2007.05.005
[36] R.A. Turner. Anticonvulsants. Screening methods in pharmacology. Amsterdam: Elsevier, 1965: 164–172. https://doi.org/10.1016/b978-1-4832-3266-9.50018-9
[37] H. Joshi, M. Parle. Zingiber officinale: Evaulation of its Nootropic effect in mice. African Journal of Traditional, Complementary and Alternative Medicines, 2006, 3(1): 64–74. https://doi.org/10.4314/ajtcam.v3i1.31140
[38] G.S. Taiwe, E.N. Bum, T. Dimo, et al. Antidepressant, myorelaxant and anti-anxiety-like effects of Nauclea latifolia Smith (Rubiaceae) roots extract in murine models. International Journal of Pharmacology, 2010, 6(4): 364–371. https://doi.org/10.3923/ijp.2010.364.371
[39] G.L. Ellman, K.D. Courtney, V. Andres, et al. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology, 1961, 7(2): 88–95. http://dx.doi.org/10.1016/0006-2952(61)90145-9
[40] I.P. Lowe, E. Robins, G.S. Eyerman. The fluorometric measurement of glutamic decarboxylase and its distribution in brain. Journal of Neurochemistry, 1958, 3(1): 8–18. https://doi.org/10.1111/j.1471-4159.1958. tb12604.x
[41] H. Ohkawa, N. Ohishi, K. Yagi. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 1979, 95(2): 351–358. https://doi.org/10.1016/0003-2697(79)90738-3
[42] P. Kakkar, B. Das, P.N. Viswanathan. A modified spectrophotometric assay of superoxide dismutase. Indian Journal of Biochemistry and Biophysics, 1984, 21(2): 130–132. http://nopr.niscpr.res.in/bitstream/123456789/19932/1/IJBB%2021%282%29%20: 130-132.pdf
[43] H.U. Bergmeyer. Methods of enzymatic analysis. Weinheim/NewYork: Verlag Chemie/Academic Press Inc., 1974: 673–680.
[44] J.D. Banchroft, A. Stevens, D.R. Turner. Theory and practice of histological techniques. Fourth Ed. Edinburgh: Churchil Livingstone, 1996.
[45] J. Panyam, V. Labhasetwar. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Advanced Drug Delivery Reviews, 2003, 55(3): 329–347. https://doi.org/10.1016/s0169-409x(02)00228-4
[46] I. Fridovich. Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? Annals of the New York Academy of Sciences, 1999, 893: 13–18. https://doi.org/10.1111/j.1749-6632.1999.tb07814.x
[47] Y. Gilgun-Sherki, E. Melamed, D. Offen. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology, 2001, 40: 959–975. https://doi.org/10.1016/s0028-3908(01)00019-3
[48] H.G. Vogel. Skin sensitization testing. In: Drug discovery and evaluation: pharmacological assays. Berlin, Heidelberg: Springer, 2014: 1–7. https://doi.org/10.1007/978-3-642-27728-3_94-1
[49] P. Kwan, G.J. Sills, M.J. Brodie. The mechanisms of action of commonly used antiepileptic drugs. Pharmacology and Therapeutics, 2001, 90(1): 21–34. https://doi.org/10.1016/s0163-7258(01)00122-x
Copyright© Jayaraman Rajangam, Pushpalatha Sampathi, Narahari Palei, Anna Balaji, Shyam Sundar A., Bibhash Chandra Mohanta and Vasanth Raj Palanimuthu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.