Research Article
Metals Concentrations in Blood and Urine Measured Using a New Nano Metals Oxide Electrode
Medical Physics Department, Hilla University College, Babylon, Iraq
* Corresponding authors. E-mail: dr.emadsalaama@hilla-unc.edu.iq
Received: Jul. 16, 2021; Accepted: Sep. 19, 2022; Published: Sep. 19, 2022
Citation: Emad Salaam Abood, Metals Concentrations in Blood and Urine Measured Using a New Nano Metals Oxide Electrode. Nano Biomed. Eng., 2022, 14(2): 107-113.
DOI: 10.5101/nbe.v14i2.p107-113.
Abstract:
One of the most appealing features of ion-selective electrodes (ISE) is their ease of use, an electrical method used to measure common elements such as sodium, potassium, and chlorine in the autoanalyzer and its development for the measurement of heavy elements such as zinc and copper through the manufacture of highly selective and sensitive electrodes. A new metals oxide carbon paste electrode (MCPE) with good ion-capturing to detect the level of heavy elements in serum and solutions was created by synthesizing the of (Zn,Cu)-O nanoparticles using a hydrothermal method. For optimal circumstances, the physical and chemical characteristics of the electrode were investigated. The cyclic voltammetry redox wave was used to record a signal. By cyclic voltammograms, a large signal for oxidation-reduction occurs with a reversible mechanism due to one electron transmission for ionization of zinc and a quasi-reversible peak for the Cu metal. Thermodynamic and kinetic aspects have also been investigated such as ∆E, ∆H, ∆G, ∆S equal to -4.32, -32.19, 33.40, 0.031 respectively. The results demonstrate that the novel electrode can discriminate between peaks produced by redox processes of two elements at a similar time, having two different oxidation peaks and two separate redaction peaks. The electrical conductivity qualities of zinc and copper allowed us to build and extend electrical cells and measure five elements simultaneously using an electrical technique.
Keyworlds: ISE, Cyclic Voltammetry, carbon paste electrode, nano metals oxide
Introduction
The activity of ions determine by the ion-selective electrode (ISE) is a technique for measuring the electrical potential of an aqueous solution. It is capable of tracking variations in ion activity over time. It can tell the difference between positively and negatively charged ions. [1] The ISE Rules are made up of a thin membrane that allows just the targeted ion to pass through. By diffusion mechanism, a through A potential difference is created by selective binding with certain locations inside the membrane. [2] Glass electrodes are determined by the chemical characteristics of a specified composition glass membrane.[3] The glass electrode has a high level of sensitivity. It may be used for a variety of purposes. [4] Where chemical inertness is critical, the glass electrode (GE) is most commonly employed. [5] Clean and calibrating with a standardized buffer solution is simple and convenient. Conduction within the dried glass is caused by the lowest-charged cation and is unrelated to penetration by a large number of cations. pH electrodes are likely to be processed by ISEs. [6] They are used for Cl-, K+, Na+, and others. [7] A pH electrode, which is sensitive to the H+ ion, is a common ISE. Using pH electrodes as an example, measuring the pH of a solution is simple after proper calibration. [8] In chemical analysis: Potentiometry is a technique based on measuring the potential between two electrodes while controlling the electric current between the electrodes. [9] Ionic activity in the test solution given species, a linear with a sloping factor provided by the Nernst equation formula eq.1. [10] The basic equation that connects the electrode potential to the observed ion activity in the test solution. [11]
![]()
Where E: constant ISE/reference,![]() : total potential (mV), R: gas constant, T: Temperature, n ionic charging, F: Faraday constant and Log(a) logarithm activity ion. [12].
: total potential (mV), R: gas constant, T: Temperature, n ionic charging, F: Faraday constant and Log(a) logarithm activity ion. [12].
The electrode has a Nernstian response if a plot of the potential difference measured against a reference electrode displays a Nernstian response for a given concentration range. [13] In this research, we used zinc and copper because of their distinctive physical, chemical, and electrical properties, Zinc is a vitamin that everyone requires for a healthy body. [14] Zinc is found in cells. [16] It helps the immune system; many diseases cause a change in the concentration of serum level. [17] As a result, a quantitative assessment of zinc level concentration is important in analytical chemistry for the development of physiological and pharmacological research. [18] Flow injection [19], fluorimetry [20], and spectrophotometry techniques are among the methods used to determine zinc content. [21] The electrochemical response of a zinc-on-zinc ion electrode was shown in certain publications. [22] Copper is required for metabolic processes in humans, and it is found in all cells. It aids in the maintenance of nerve cells, the production of red blood cells, and the overall functioning of our immune systems. [23], we created a very sensitive and selective method using the cheapest ion-selective electrode to determine the trace metal concentrations in various solutions by sensing the small change in current during the oxidation-reduction process when a potential overworking electrode was used.
Experimental
Metal oxide nanoparticles preparation by hydrothermal methods
Zinc oxide nanoparticles (ZnNPs) and copper oxide nanoparticles (CuNPs) were synthases by a used hydrothermal method when used 0.3 g from ZnCl2 and dissolved in 50 ml of Distilled water (D.W) with continuous string, 10 ml from NaOH 0.1 M added to the burette and controlled in a very slowly dropping addition to preventing side product, after 10 h; a white precipitate is formed, washed by D.W and dissolved a gene in a solution containing 6 g from polyvinylpyrrolidone (PVP) to get a homogenous solution, after that transfer to the oven with heated to 433 K for overnight, the CuO-NPs dark prawn powder was prepared as the same method, Shaw the TEM is seen in Figs. 1 and 2. ( INSPECT S50, Tehran University, Iran) This approach was used to determining the shape of the molecules created. Samples of ZnO-NPs and CuO-NPs
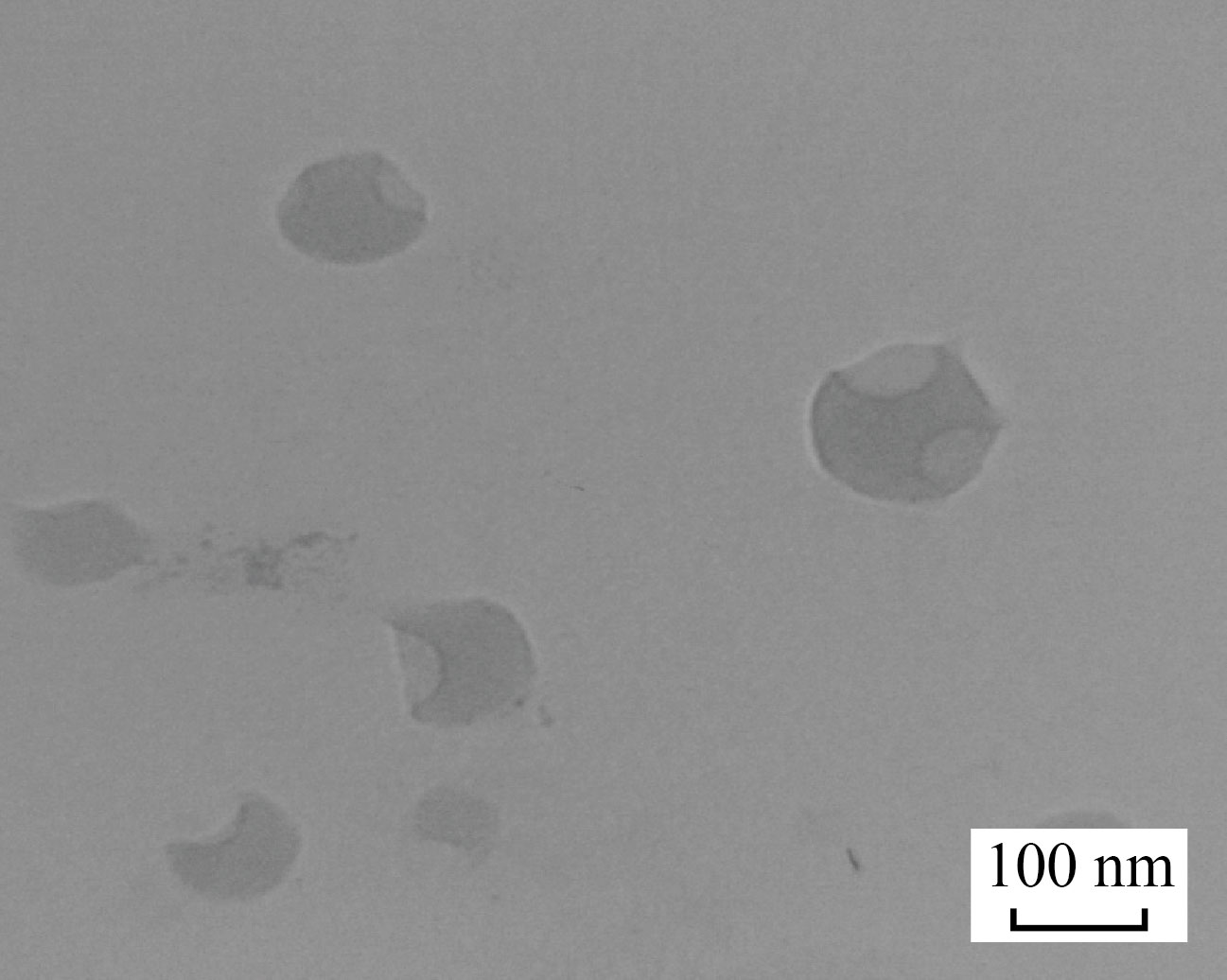
Fig. 1 TEM of ZnO-NPs
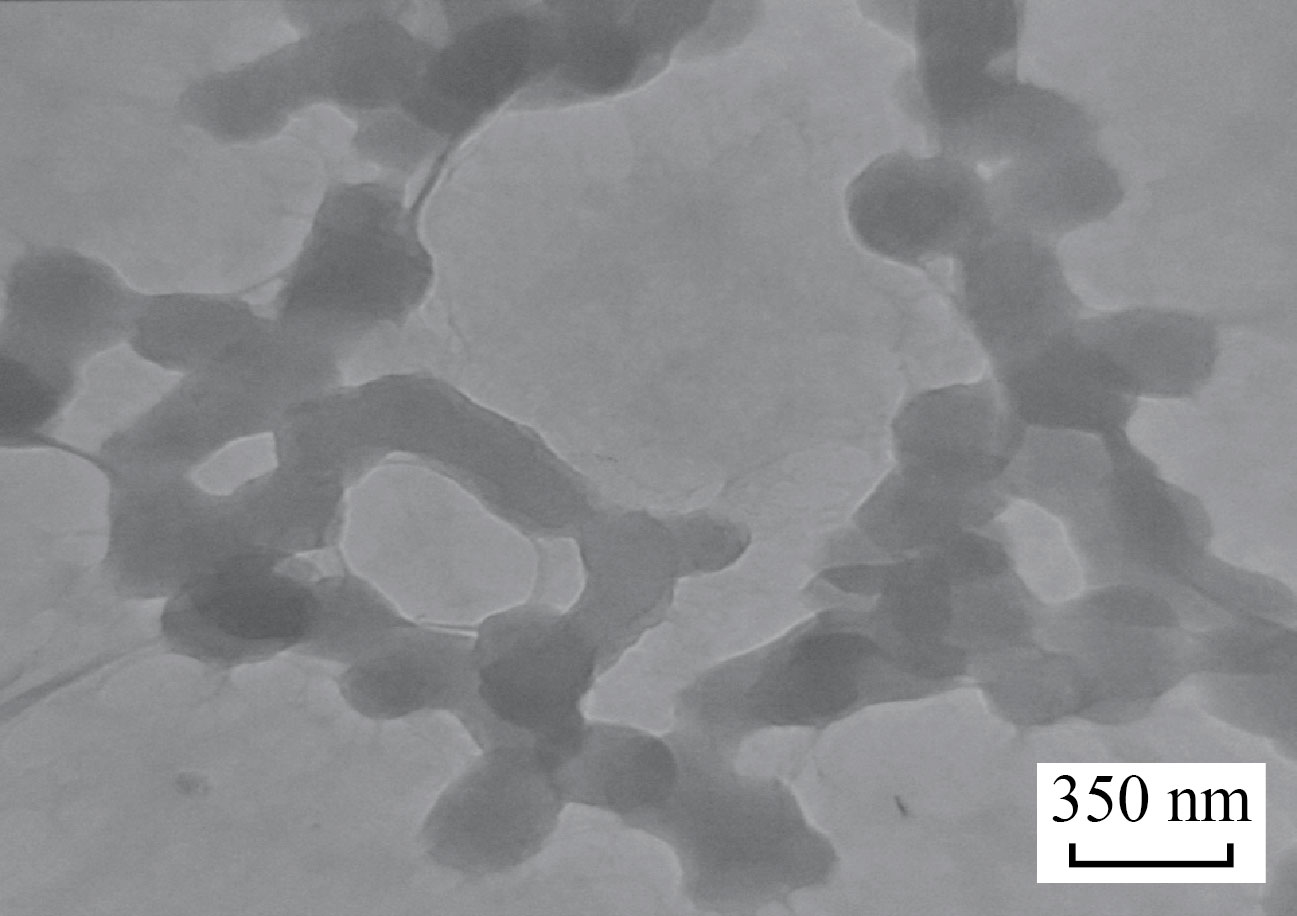
Fig. 2 TEM of CuO-NPs
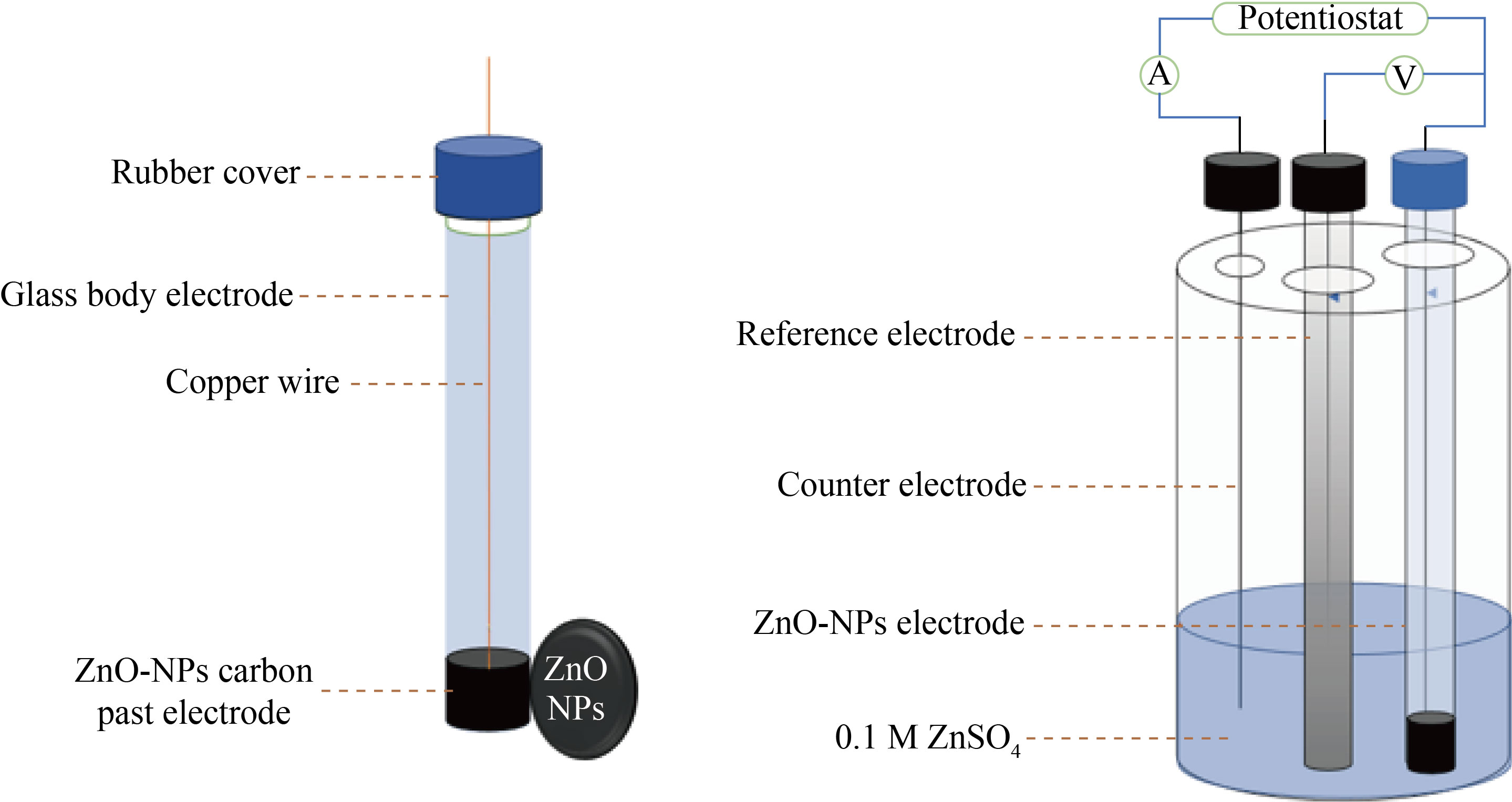
Fig. 3 ZnO-NPs-CPE in cyclic voltammetry Cell
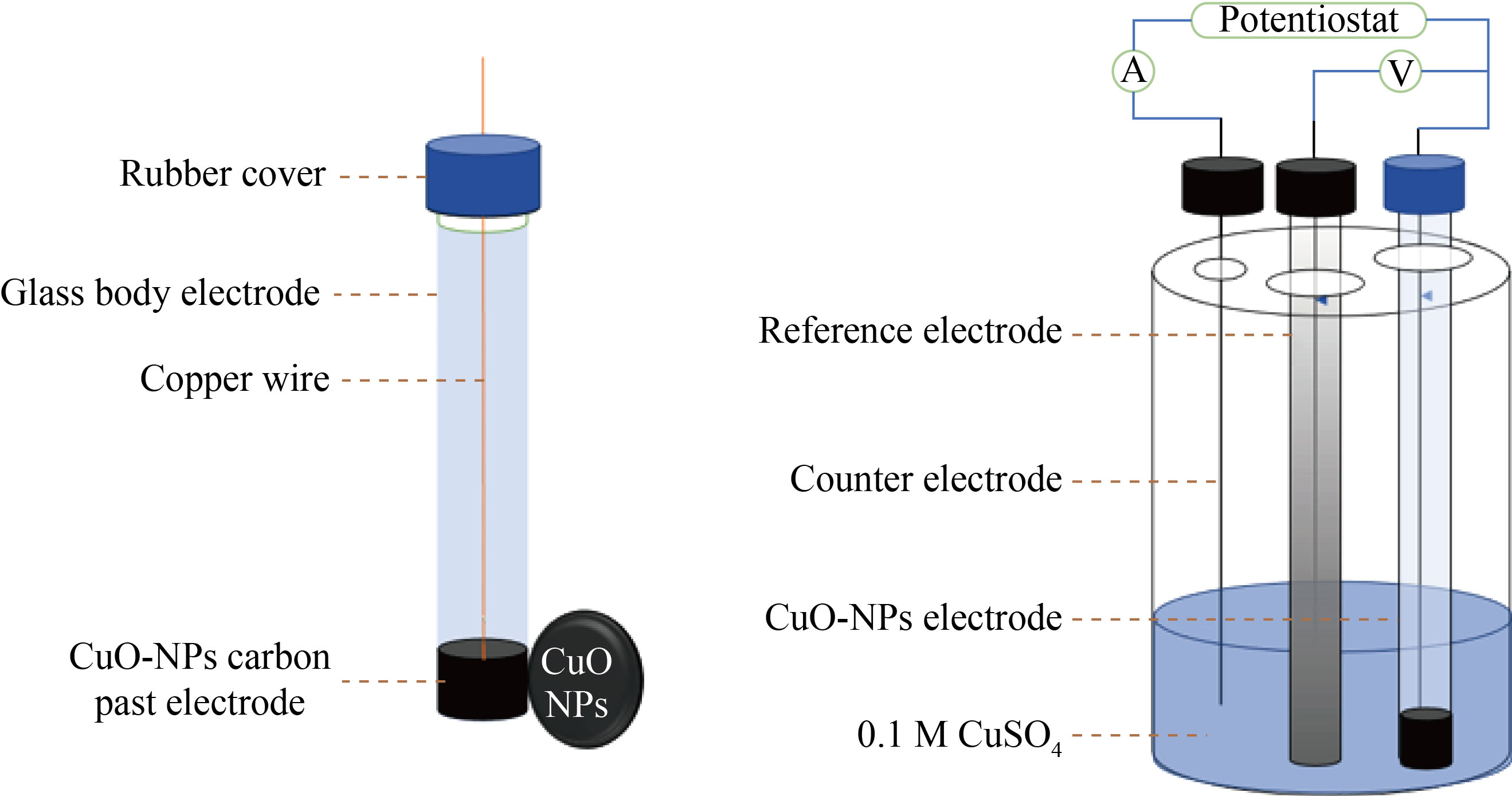
Fig. 4 CuO-NPs-CPE in cyclic voltammetry Cell
Result and Discussion
Preparation of (ZnO-NPs and CuO-NPs) carbon paste electrode
The ZnO-NPs-CPE was made by combining 0.2 g, 0.5 g, and 0.4 mL of ZnO-NPs, using a mortar, combine graphite powder and paraffin oil until a black paste is formed. One end of a glass tube is filled with paste (0.131 mm2 cross-sectional area, 3 cm length). Using a copper wire to produce electrical conductivity in the carbon paste, The surface of the carbon paste was smoothed with fine paper. The blank CPE was made in the same method as the other CPEs, but without the addition of ZnO or CuO-(NPs) to the paste. In the same, way prepared the (CuO-NPs-CPE) fig 3 and 4 show the scheme of the body electrode.
The signal was recorded by Digi-ivy 2113, USA, potantiostatic which was controlled with system software.
Cyclic voltammetry study of Zn and Cu ISE.
The mechanism of reaction:
When potentials are applied over the electrode in electrochemical cell reactions, the supporting electrolyte works to balance charge distribution in solution, and the axillary electrode was used to record the cyclic voltammetry signal when metals ions attached to the electrode surface and electrons transferred to show oxidation and reduction peaks by cyclic voltammograms.
Zinc
Fig. 5a cyclic voltametric electrochemical redox reaction response of 0.3 M ZnSO4 at ZnO-NPs-CPE. The oxidation and reduction peaks of Zn+2 (-1.061 V and -0.810 V), respectively. C.V shown a very apparent. for the ZnO-NPs-CPE with ZnSO4 solution because the Fig.5b refers to CPE voltammogram without response when the applied potential at the same solution. Scan rate affected study on the electro-redox of zinc was investigated by (CV) (Fig. 6) show in the region of 0.1-5 V/s, a peak of current (Ip) vs (V1/2) was linear. Diffusion was used instead of surface control.
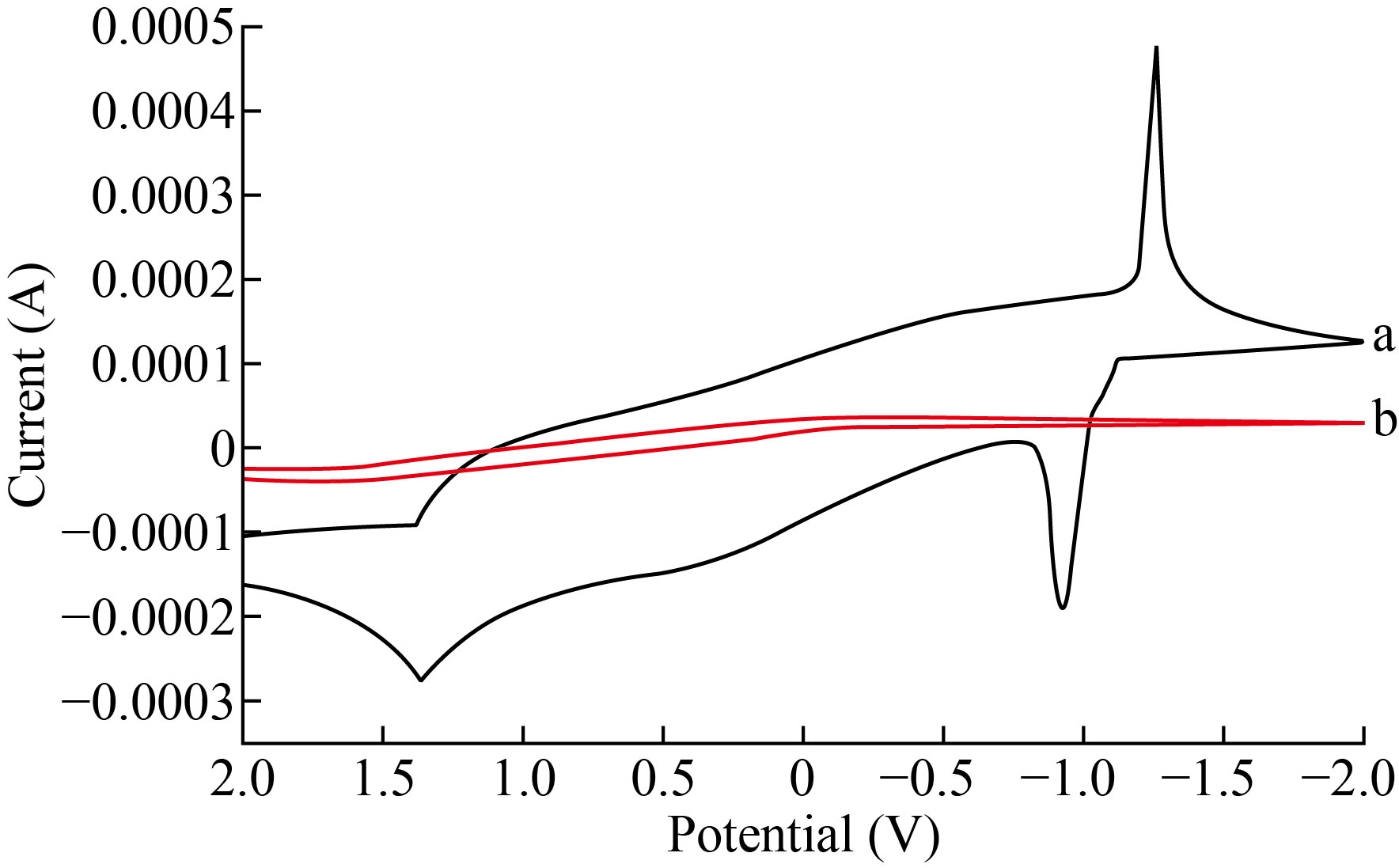
Fig. 5 a: refer to signal response for zinc ion by ZnO-NPs-CPE b: non-response for CPE
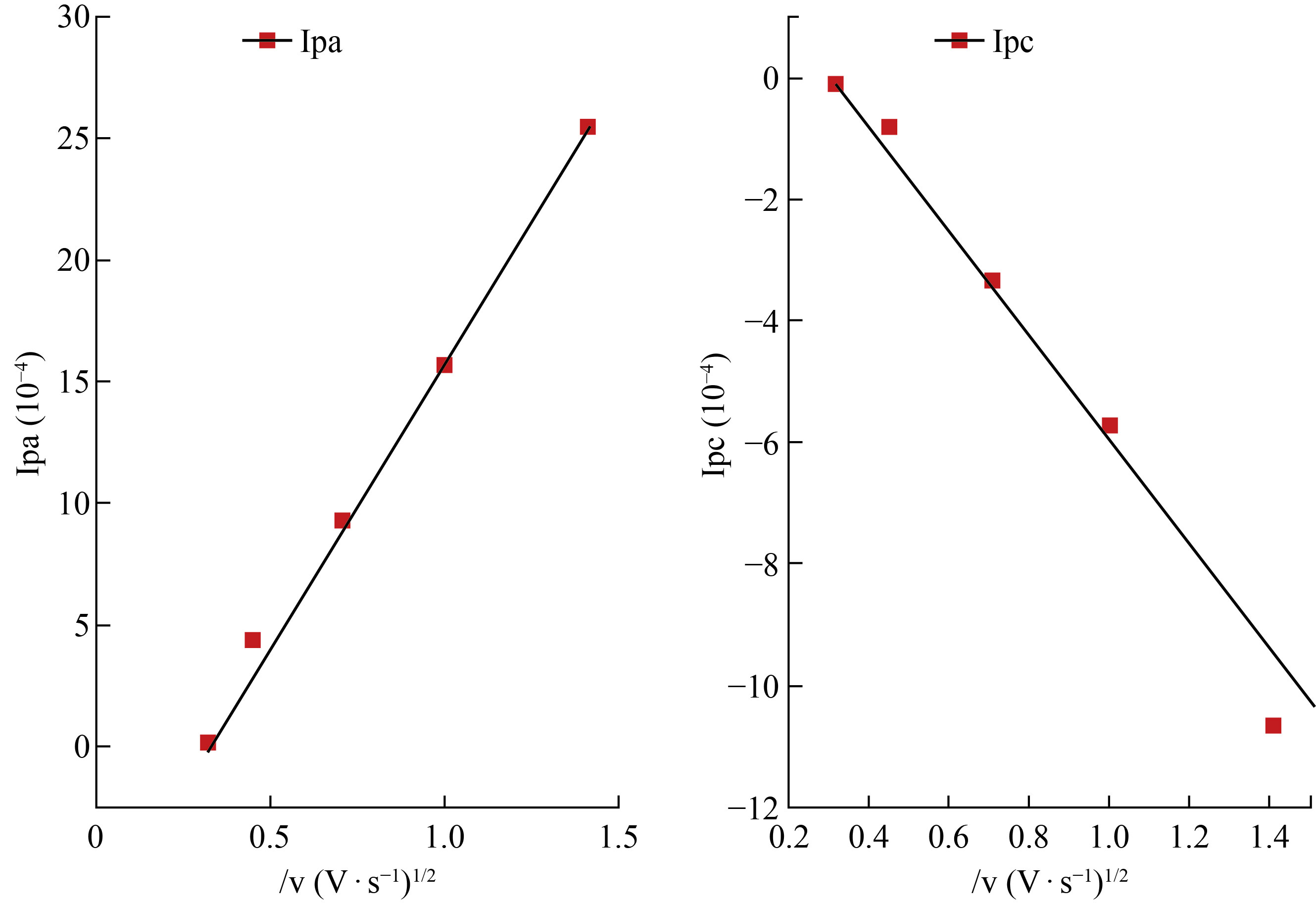
Fig. 6 Ipoxd and Ipred / ʋ1/2 for Zn+2.
ZnO-NPs-CPE with effect of Temperature
Redox with CV at temperatures ranging from 15 to 30 degrees Celsius using a calomel reference electrode; The cyclic voltammogram used to determination of activation energy. Charge-transfer resistance dependence of temperature and During the electro-oxidation process, a diffusion intermediate increases the rate were inextricably linked to the amount of surface covering. Result refers to the working electrode tack the clear signal but the beaks shift about Nernst condition except 25oC, by used equation lnD = ln Do - ![]()
Where: D, T and K (diffusion coefficient, temperature and Temperature in kelvin) respectively.
Fig.4 the curve between ![]() , the free energy was estimated using this equation and found to be Ea = 48.11 KJ/mol. And use Equation
, the free energy was estimated using this equation and found to be Ea = 48.11 KJ/mol. And use Equation ![]() to calculate the exothermic process that occurs when potential is applied to the ZnO-NPs-CPE.
to calculate the exothermic process that occurs when potential is applied to the ZnO-NPs-CPE.
Where: ∆G, F, R and Ep ( free energy, Faraday constant, gas constant, and difference in potential) respectively .
∆G was found to be 49.2 KJ/mol, indicating that it was a spontaneous reaction. And by used Nicholson equation Eq. ![]() , the constant of reaction Kp was calculated and inserted in table 1 with other physical and chemical parameters
, the constant of reaction Kp was calculated and inserted in table 1 with other physical and chemical parameters
A quasi-reversible cyclic voltammetry reaction with a first-order reaction is the mechanism of the reaction on the electrode surface. The physical parameters characteristics for the zinc oxide electrode are shown in Table 1.
Table 1 Inset shows the results of ZnO-NPs-CPE in the presence of 1x10-1 M ZnSO4 solution at scan rates of 0.1, 0.2, 0.5, 1, 2, and 5 V.S-1.
Ipa/Ipc | Fpred A/(v.s-1)1/2 (10-4) | Ipred A (10-4) | Epc | FpOX A/(v.s-1)1/2 (10-4) | Ipox A (10-4) | Epa (V) | √ʋ (v.s-1)1/2 | ʋ V.Sec-1 |
0.804 | -0.235 | -0.199 | -0.422 | 0.437 | 0.160 | -0.642 | 0.316 | 0.1 |
1.990 | -0.472 | -1.503 | -0.402 | 8.725 | 2.992 | -0.780 | 0.447 | 0.2 |
3.242 | -3.595 | -2.220 | -0.336 | 12.051 | 7.198 | -0.835 | 0.707 | 0.5 |
3.903 | -3.738 | -3.638 | -0.299 | 14.590 | 14.590 | -0.849 | 1.000 | 1.0 |
2.445 | -6.499 | -9.559 | -0.163 | 17.700 | 23.380 | -1.198 | 1.410 | 2.0 |
Standard curve by ZnSO4 with ZnO-NPs-CPE.
To identify the limits of Zn2+, a calibration curve was created. It was investigated if ZnO-NPs-CPE could separate the electrochemical response of Zn2+. As a result, CV was utilized to record varied current quantities in order to determine the electrode's response to various concentration standard solutions in natural medium pH = 7.0. The ZnO nanoparticle's reaction to Zn was clearly revealed by the CV calibration curve, which was produced at 1-10 ppm. All results show the best signal and clarify response of ZnO-NPs-CPE was more sensitive and selective. Fig7 shows the calibration curve by cyclic voltammograms with a curve plot.
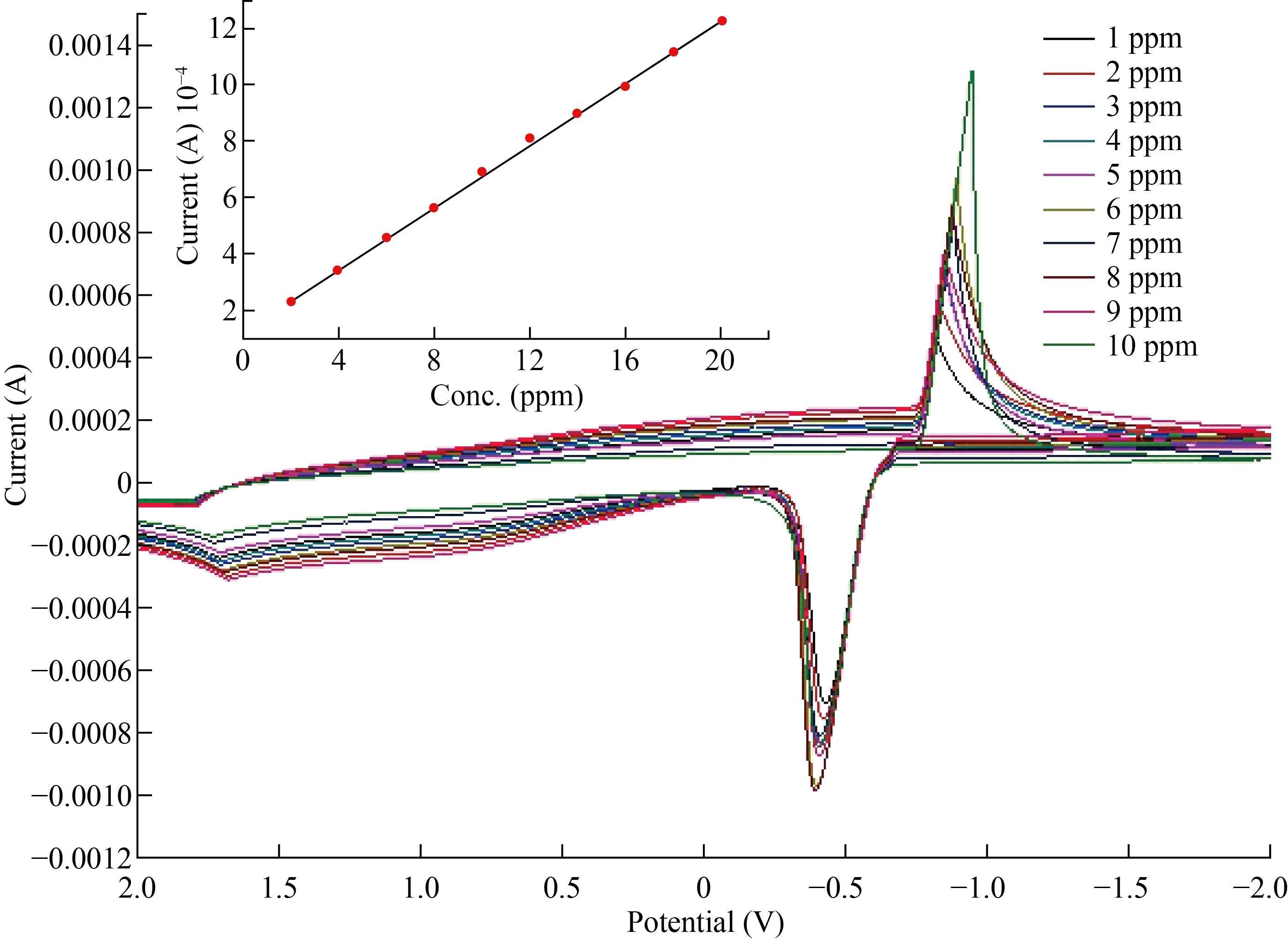
Fig. 7 Calibration curve and plot curve for the Zn+2 by used standard ZnSO4 solutions ZnO-Ns-CPE
Copper
Figure 8 shows the cyclic voltammetry response of 0.1 M CuSO4 electrochemical redox at CuO-NPs-CPE. Cu2+ oxidation had Epa of around 0.598 V, while Cu2+ reduction had an Epa of about 1.098 V. In other words, the result clearly indicated by CuO-NPs-CPE improved the copper redox signal.
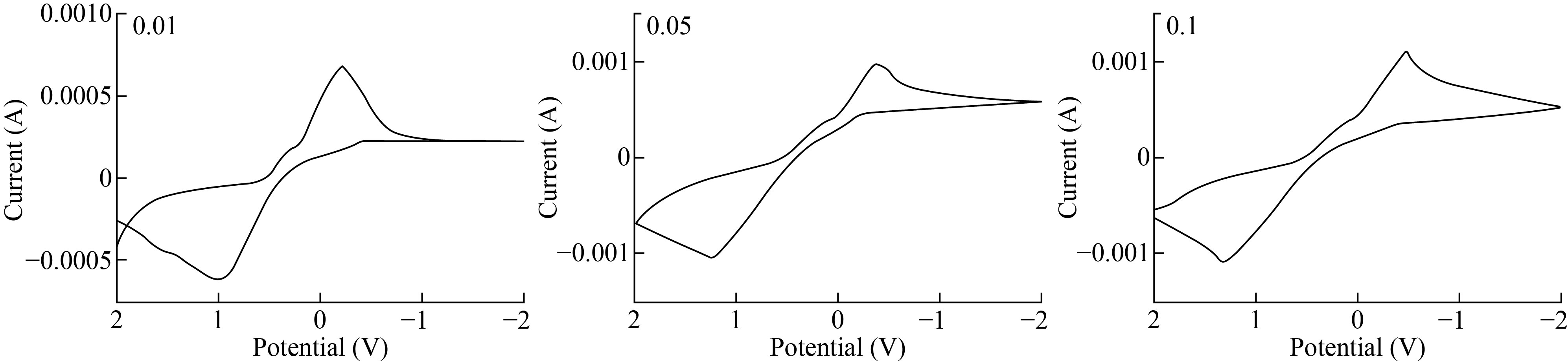
Fig. 8 C.V by CuO-NPs-CPE with some level of solutions conc.
The variation curves allow for the Eactivity across a large range of potentials to choose the physical and chemical characteristics that are inserted in table 2 with the optimum condition for electrode operating with a calomel electrode at temperatures ranging from 15 to 35 degrees Celsius. The cyclic voltammograms at varied temperatures are shown in Fig. 9.
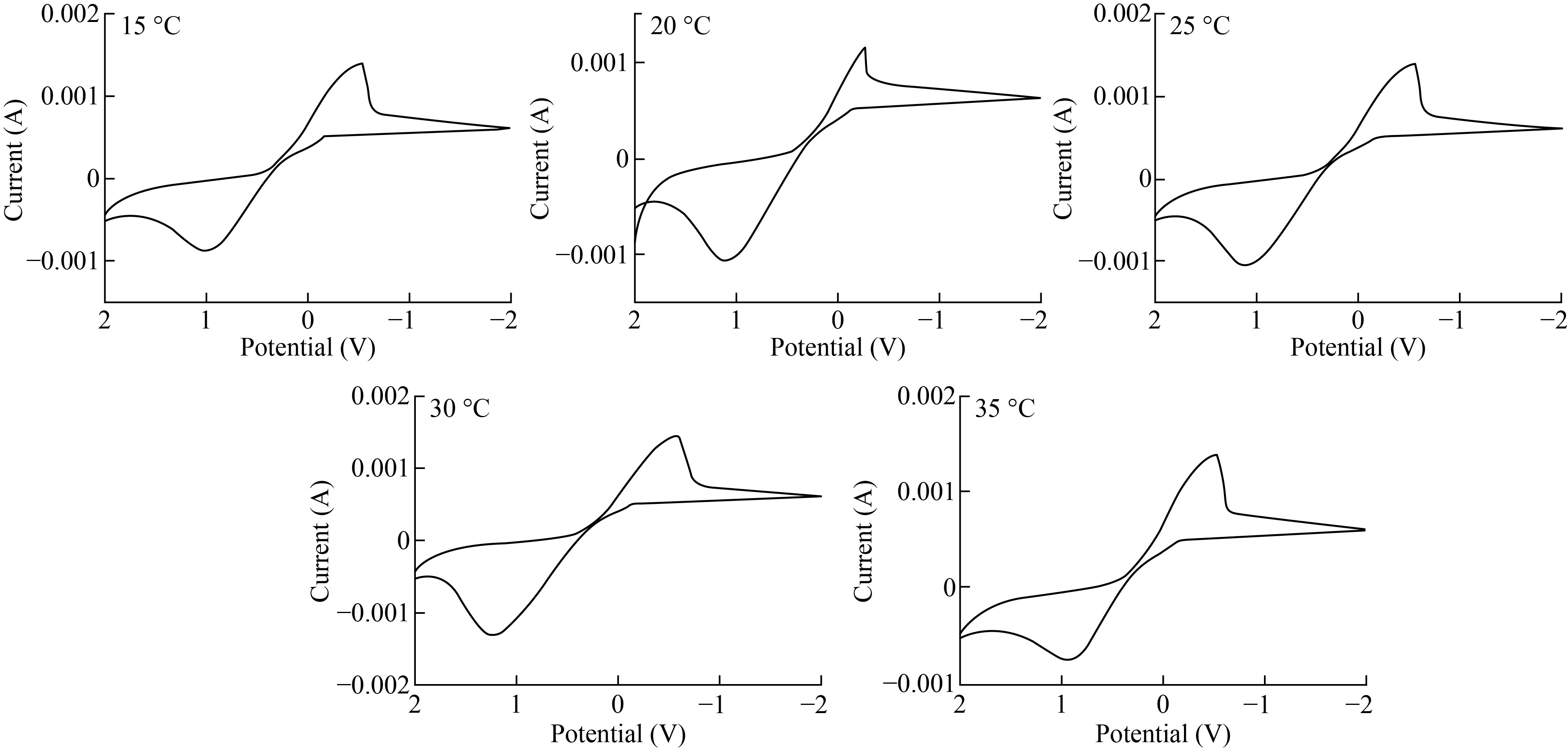
Fig. 9 The impact of temperature on the cyclic voltammograms of CuO-NPs-CPE at temperatures of 15, 20, 25, 30, and 35 oC, respectively.
Table 2 The thermo-kinetic parameters for the CuO-NPs-CPE
| ∆E KJ/mol | ∆H KJ/mol | ∆G KJ/mol | ∆S KJ/mol | Keq cm.S‑1 | Do 10-4 | Α
| K 10-3 |
CuO-NPs-CPE | -4.32 | -32.19 | 33.40 | 0.031 | 7.8-10-7 | 61.8 | 0.25 | 0.49 |
Standard curve by CuSO4 with CuO-NPs-CPE
CuO-NPS-CPE was used to investigate the electrochemical response of Cu+2. Fig. (10) displays the calibration plot curve obtained via CV in (0.2 – 2) ppm by changing the content of the CuSO4 pure stock solution. It is obvious that the response to Cu+2 is a positive one
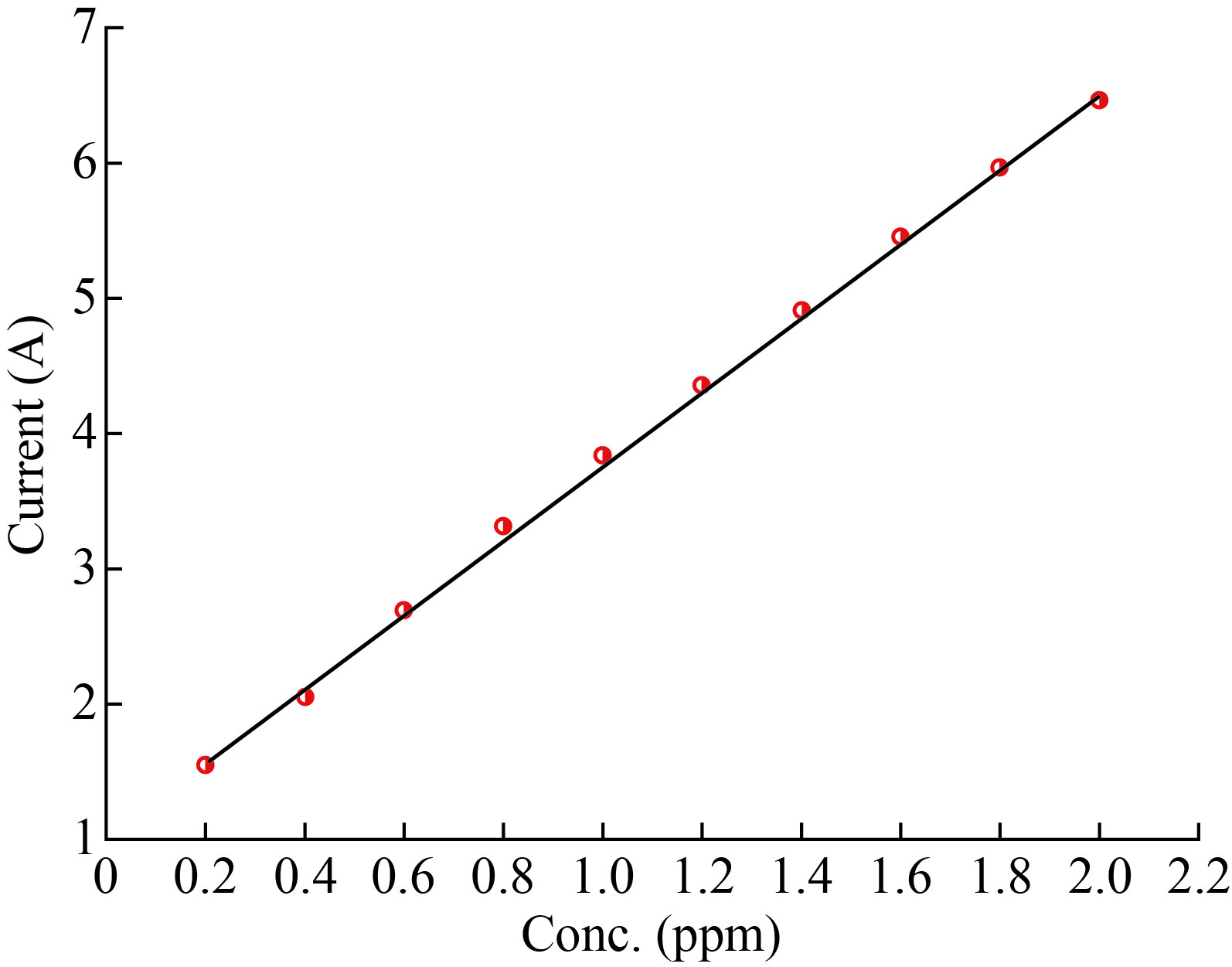
Fig. 10 Calibration plot curve for the standard solutions by CuO-NPs-CPE
Conclusions
The hydrothermal method was successful in preparing ZnO and CuO nanoparticles, and cyclic voltammetry is the best technique for detecting metals in electrochemical reactions, metals oxide with graphite powder and paraffin oil tacks a highly sensitive paste membrane to use for selective electrodes. Finally; metals oxide ion-selective electrodes were prepared and a trace concentration of metals determined in various solutions.
Recommendation
The researchers recommend medical device engineers design an electrode body that matches the electrodes used in the electrolyte auto analyzer with connecting them in the electrical circuit to be measured at the same times. The conclusion of the research and through the conditions that have been studied shows that the measurement of heavy metals removes many obstacles from solutions used, costs and, error rates found in other common techniques.
Conflict of Interests
The authors declare that no competing interest exists.
References
- G. Bannach, P. Cervini, É.T.G. Cavalheiro, using thermal and spectroscopic data to investigate the thermal behavior of epinephrine. Thermochimica Acta, 2010, 499: 123-127.
- Dawadi, S., Gupta, A., Khatri, M. et al. Manganese dioxide nanoparticles: synthesis, application and challenges. Bull Mater Sci 43, 277 (2020). https://doi.org/10.1007/s12034-020-02247-8
- P. Xie, X. Chen, F. Wang, et al., Electrochemical behaviors of adrenaline at acetylene black electrode in the presence of sodium dodecyl sulfate. Colloids and Surfaces B: Biointerfaces, 2006, 48, 17-23.
- G. Crespo, School of Chemical Science and Engineering, Applied Physical Chemistry Division, KTH Royal Institute of Technology, Electrochimica Acta, Volume 245, 10 August 2017, Pages 1023-1034.
- Awwad, A. M., Amer, M. W., Salem, N. M., & Abdeen, A. O. (2020). Green synthesis of zinc oxide nanoparticles (ZnO-NPs) using Ailanthus altissima fruit extracts and antibacterial activity. Chem. Int, 6(3), 151-159.
- P. Pradhan, R.J. Mascarenhas, T. Thomas, et al., Electropolymerization of bromothymol blue on carbon paste electrode bulk modified with oxidized multiwall carbon nanotubes and its application in amperometric sensing of epinephrine in pharmaceutical and biological samples. Journal of Electroanalytical Chemistry, 2014, 732: 30-37.
- C. Hu, S. Hu, H. Yi, et al., Functionalized multiwalled carbon nanotubes through in situ electropolymerization of brilliant cresyl blue for determination of epinephrine. Electroanalysis, 2010, 20: 1143.
- H. Nakazawa, Y. Ishii, M. Iijima, et al., Determination of nitrotyrosine and tyrosine by high-performance liquid chromatography with tandem mass spectrometry and immunohistochemical analysis in livers of mice administered acetaminophen. Journal pharma Biomed, 2015, 41: 1325.
- Baghdadi, Y. N., Youssef, L., Bouhadir, K., Harb, M., Mustapha, S., Patra, D., & Tehrani‐Bagha, A. R. (2020). The effects of modified zinc oxide nanoparticles on the mechanical/thermal properties of epoxy resin. Journal of Applied Polymer Science, 137(43), 49330.
- Asadollahzadeh, Hamideh. "Developing an electrochemical sensor based on a carbon paste electrode modified with ZnO nanoparticles synthesized by microwave for determination of chlorpheniramine maleate." Analytical Methods in Environmental Chemistry Journal 2021: 4.01: 16-25.
- J.X. Du, L.H. Shen and J. R. Lu, Cetyltrime thylammonium bromide-enhanced chemiluminescene determination of uric acid using a luminal-hexacyano ferrate (III) system. The Japan Society for Analytical Chemistry, 2003, 21: 183.
- F.N. Chen, Y.X. Zhang, and Z.J. Zhang, Simultaneous determination of epinephrine, noradrenaline and dopamine in human serum samples by high performance liquid chromatography with chemiluminescence detection. Chinese Journal of Chemistry, 2007, 25: 942-946.
- M.H. Sorouraddin, J.L. Manzoori., E. Kargarzadeh, et al., Electrochemical application to biology. Electrochemical Society, 2009, 08534-2839.
- Anantha, M. S., et al. "ZnO@ MnO2 nanocomposite modified carbon paste electrode for electrochemical detection of dopamine." Sensors International 2 (2021): 100087.
- A.A.A. Darwish, M. Rashad, A.E. Bekheet, et al., Linear and nonlinear optical properties of GeSe2-xSnx thin films for optoelectronic applications. Journal of Alloys and Compounds, 2017, 709: 640-645.
- R.L. Penn, J.F. Banfield, ZnO-based dye-sensitized solar cells. Journal of Alloys and Compounds, 2017, 91, 1685- 1689.
- Abood, Emad Salaam, Amer Mousa Jouda, and Muthana Saleh Mashkoor. "Zinc metal at a new ZnO nanoparticle modified carbon paste electrode: a cyclic voltammetric study." Nano Biomed Eng 10.2 (2018): 149-155.
- Abood, Emad Salaam, Mothana Salih Mashkoor, and Amer Mousa Jouda. "Cyclic Voltammetry Study for MnO2 Nanoparticles Modified Carbon Paste Electrode." Nano Biomed. Eng 11.4 (2019): 368-374.
- Abood, E. S. (2021). Mn Determination by Metals Oxide Nanoparticles Mix Ions Selective Carbon Paste Electrode Industrialization and Cyclic Voltammetry Application Study. Nano Biomed. Eng, 13(3), 249-256.
- O. Fasakin, M.A. Eleruja, O.O. Akinwunmi, et al., Synthesis and characterization of metal organic chemical vapour deposited copper titanium oxide (Cu-Ti-O) thin films from single solid source precursors. Journal of Modern Physics, 2013, 4: 41158.
- Jafat, R., Abood, E. (2022). 'Corrosion Inhibition Of Low Carbon Steel Using Green Inhibitor (Bulrush) At Variable Conditions', Egyptian Journal of Chemistry, 65(2), pp. 565-570.
- Moghaddas, S. M. T. H., Elahi, B., & Javanbakht, V. (2020). Biosynthesis of pure zinc oxide nanoparticles using Quince seed mucilage for photocatalytic dye degradation. Journal of Alloys and Compounds, 821, 153519.
- Abood, E., Abed, A., Salman, Z. (2021). 'New Fe2O3 Nanoparticles Modified Carbon Paste Electrode: A Cyclic Voltammetric Study', Egyptian Journal of Chemistry, 64(12), pp. 7509-7515.
Copyright© Emad Salaam Abood. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.